Gastrointestinal stromal tumors (GIST-GIST) are quite rare (15 to 20 cases per million people).
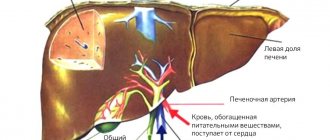
Most often, stromal tumors appear in the stomach (about seventy percent of all cases), less often in the small intestine, duodenum, colon and rectum, and esophagus.
GIST tumors can develop regardless of age, but in approximately seventy-five percent of cases they are found in patients over fifty years of age. Stromal tumors are very rare in children.
Symptoms of the disease
In most patients, GISTs are discovered incidentally during routine examinations (gastroscopy or colonoscopy, computed tomography or during surgery), since they do not cause any complaints for a long time.
As a rule, the first symptoms appear when a fairly large tumor begins to compress adjacent organs and tissues, however, these complaints are nonspecific and may have a completely different, non-tumor cause. However, if any complaints arise that persist for a long time, you should contact a specialist to undergo diagnostics in Germany and clarify the diagnosis.
The main symptoms of gastrointestinal stromal tumors include:
- In the esophagus Increasing complaints when swallowing as the size of the tumor increases, resulting in problems with eating.
- With a GIST tumor in the stomach or duodenum, nausea (often, but not always), stomach pain or a feeling of rapid satiety in the stomach.
- In the small intestine Constipation, “black” stool (a sign of possible bleeding in the intestines caused by tumor processes).
- In all cases, weight loss is due to metabolic disorders in the body. Anemia due to hidden bleeding caused by the tumor and insufficient supply of nutrients, resulting in severe weakness and general exhaustion of the body.
Symptoms of stromal tumors
Symptoms of GIST are similar to other gastrointestinal tumors and may include:
- Hidden bleeding;
- Gastrointestinal bleeding;
- Intestinal obstruction;
- Abdominal enlargement;
- Feeling of quick satiety;
- Pain;
- Weakness.
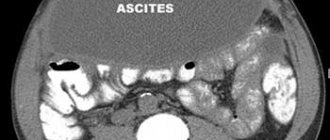
It is especially worth noting that patients often (from 10 to 25% of cases, depending on the type of tumor) experience metastasis of stromal tumors to the liver, peritoneum, regional lymph nodes, lungs, soft tissues, and rarely to the brain.
get a free consultation
Diagnosis and staging of tumor
When deciding on the tactics and method of treatment, an important element is a thorough diagnosis of cancer, aimed at establishing an accurate diagnosis and determining the extent of spread of the disease in the body. Detailed information on the diagnosis of GIST is presented in the table below:
| Surveys | Notes |
| Physical examination | |
| Blood analysis | To assess organ function (complete blood count, renal and liver parameters, coagulation, TSH) |
| Endoscopy, endosonography | To determine the pattern of spread of the disease, as well as to obtain biomaterial for histological confirmation of the diagnosis |
| CT scan of the chest, abdomen, pelvis (with contrast) |
|
| PET-CT | (in some cases) To confirm the diagnosis and determine the stage of the disease |
| Histology |
|
| Molecular genetics | Mutations of the KIT/PDGFR gene at the primary diagnosis, over time with disease resistance to therapy |
Benefits of treatment in Belgium
- Availability of the latest drugs. In Belgium, if the tumor is resistant to therapy with imatinib, sunitinib and regorafenib, the patient has the opportunity to receive treatment with new drugs - sorafenib, dasatinib and nilatanib. This helps prolong life even in the most difficult cases.
- Laparoscopic minimally invasive surgery. It is used in Belgium for tumors not exceeding 5 cm, located in the stomach and small intestine. Advantages of laparoscopic resection include faster resumption of normal nutrition, shorter hospital stay after surgery, and less use of analgesia. The short-term results remain the same as with open surgery.
- Cost of treatment. It is approximately 30% lower in Belgium than in neighboring European countries and 60% lower than in the United States. Taking into account the high cost of drugs, this fact is of particular importance.
Prognostic factors
The most important clinical prognostic factors are mitotic index, tumor size, and location of the primary tumor. To assess the likelihood of metastases occurring, a distinction is made between possible risk categories, which are summarized in the table below:
| Risk of disease progression/recurrence | |||||||||
| Number of mitoses | Size(cm) | Stomach | % | duodenum | % | Small intestine | % | Rectum | % |
| ≤ 5 at 50 HPF | ≤ 2 | no risk | 0 | no risk | 0 | no risk | 0 | no risk | 0 |
| >2 ≤ 5 | very low | 1,9 | short | 8,3 | short | 4,3 | short | 8,5 | |
| >5≤ 10 | short | 3,6 | high | 34 | average | 24 | high | 57 | |
| > 10 | average | 12 | high | 52 | high | 52 | |||
| >5 at 50 HPF | ≤ 2 | no risk | 0 | no info | — | high | 50 | high | 54 |
| >2 ≤ 5 | average | 16 | high | 50 | high | 73 | high | 52 | |
| >5≤ 10 | high | 55 | high | 86 | high | 85 | high | 71 | |
| > 10 | high | 86 | high | high | 90 | high | |||
Symptoms
Up to 75% of GISTs are detected when they are less than 4 cm in diameter and are either asymptomatic or associated with nonspecific symptoms. They are often diagnosed incidentally during X-ray examinations or endoscopic or surgical procedures performed to investigate gastrointestinal disease or to treat emergency conditions. In Japan, mass screening for gastric adenocarcinoma using upper endoscopy has led to an increase in incidental detection of asymptomatic GISTs.
In a population-based study, the average size of GIST tumors that were detected as incidental findings was 2.7 cm, compared with 8.9 cm for those that were detected based on symptoms.
Upper gastrointestinal bleeding is the most common clinical manifestation of GIST. It is observed in 40-65% of patients. Patients who have experienced significant blood loss may report malaise, fatigue, or shortness of breath on exertion.
Obstruction may result from intraluminal growth of an endophytic tumor or from luminal compression due to an exophytic lesion. Obstructive symptoms may be organ-specific (for example, difficulty swallowing with an esophageal tumor, constipation with a rectal tumor, obstructive jaundice with a duodenal tumor).
Other symptoms:
- abdominal pain;
- anorexia;
- nausea;
- vomit;
- weight loss;
- epigastric pressure;
- fast saturation.
GIST therapy methods
The optimal cancer treatment strategy in Germany for diagnosed gastrointestinal stromal tumors has two goals: locoregional tumor control and prevention/treatment of distant metastases. The strategy also depends on tumor stage, prognostic factors (histology, mitotic index, tumor size and location), as well as patient-specific factors. An approximate algorithm of actions is presented in Diagram 1 below.
Active Surveillance
Treatment of small gastrointestinal stromal tumors of the stomach (<2 cm) is associated - after tumor resection - with a very small likelihood of recurrence, and therefore in selected cases (for example, age, comorbidity, perioperative risks) the option of regular monitoring and monitoring of the course may be considered illness (watch-and-wait strategy). In this case, the important conditions are that the tumor does not exceed 2 cm, and during endoscopic/endosonographic monitoring (every 3-6 months) no significant growth of GIST is observed. Small neoplasms with other localization (especially in the rectum) often tend to recur (to the appearance of metastases), and therefore, as a rule, they must first be removed surgically.
Surgery
Depending on the condition of the tumor and the characteristics of its development, one of the options for surgical intervention is selected.
- Surgical treatment of the primary tumor If the primary GIST initially appears resectable, primary tumor resection is indicated. As a rule, in case of a gastric tumor, this intervention is performed by wedge resection, as far as possible within 1-2 cm of healthy tissue. If necessary, segmental resection (of the small or large intestine) or en-bloc resection (of the omentum) may be indicated. As a rule, lymphadenectomy is not indicated because gastrointestinal stromal tumors rarely metastasize to the lymph nodes. If R0 resection is not possible or a very extensive operation is required, then preoperative (neoadjuvant) therapy with Imatinib is first indicated.
- Surgical treatment of metastases There are no data from prospective studies on resection of metastases. Some, but not all, retrospective studies have shown a more favorable prognosis in patients who undergo secondary resection, mainly after neoadjuvant Imatinib therapy and with a good response to treatment. If complete resection of the tumor/metastasis is possible, it should be performed during a period of good disease response to therapy (partial remission or stable disease). However, even with minimal progression of the disease during drug therapy, the prognosis of the disease worsens significantly, and with general progression of the disease, surgery is not indicated due to unfavorable prognosis. It should be noted that drug therapy must be continued even after complete removal of the metastases/tumor.
- Imatinib induction therapy To date, randomized prospective studies have not shown that resection of residual tumor manifestations after Imatinib induction therapy provides a better prognosis for patient disease. However, retrospective studies indicate that secondary resection is associated with a better prognosis when this surgical treatment in Germany is performed in patients who respond well to therapy (tumor regression or stable disease), in some cases even with focal tumor progression, as well as provided that R0 resection of the tumor/metastases can be performed. In case of multifocal progression and/or pre-predicted R2 resection, elective resection of the tumor/metastases is not indicated.
Radiation therapy
Possible palliative indications for radiation therapy include the treatment of rare bone metastases or resectable GISTs in unfavorable locations (eg, rectum or esophagus) when the disease does not respond to medical therapy.
Drug therapy
Depending on the characteristics of the tumor and its response to the treatment, the most effective option for drug therapy is selected.
- Neoadjuvant therapy (Imatinib) If complete resection of the primary tumor is not possible due to its size and/or location, preoperative (neoadjuvant) therapy with the drug Imatinib is first indicated to reduce the size of the tumor. It should be noted that in order to determine the prognostic factor for disease response to the upcoming treatment with Imatinib, it is important to determine the KIT/PDGFRA genotype, and therefore GIST cells are examined for the absence/presence of gene mutations. With the PDGFRA-D842V mutation, which makes the tumor resistant to Imatinib, neoadjuvant therapy is not indicated, the same trend is present in KIT-Wildtyp tumors. During Imatinib therapy, monitoring is carried out every 3-4 months. Once remission is achieved, tumor resection is indicated (see above for details), usually within 6-12 months.
- Adjuvant therapy (Imatinib) Adjuvant (postoperative) therapy is indicated for patients at significant risk of recurrence (see table above), while postoperative drug therapy is not indicated for patients at low risk of recurrence. Studies have shown that Imatinib therapy for 36 months (versus 12 months) significantly improves relapse-free survival, although the exact duration of adjuvant therapy is still a topic for further research and must be decided on a patient-by-patient basis. The study of KIT/PDGFRA gene mutations is an important element in deciding on adjuvant therapy. On the one hand, the determination of mutations is a prognostic factor, and on the other hand, not all mutations respond equally/equally to Imatinib therapy, which is why it is necessary to conduct a thorough analysis of tumor cells for the presence/absence of mutations.
- Additive therapy with Imatinib after metastasectomy After metastasectomy, most patients in the absence of drug therapy experience disease progression within several months, and therefore Imatinib therapy is indicated for patients after metastasectomy. This also applies to patients who received preoperative Imatinib therapy and did not have GIST progression. There is currently no optimal duration of such treatment, but, as a rule, Imatinib therapy is continued until disease relapse is detected.
- Metastatic gastrointestinal stromal tumor For metastatic GIST, drug therapy is the first choice therapy. A detailed algorithm for drug treatment is presented in Scheme 2 below.
Causes and risk factors
Scientists have discovered that cells can grow uncontrollably as a result of a disruption in their DNA. In GIST, a specific mutation causes a cellular enzyme known as KIT to be constantly turned on.
KIT is an enzyme (from the group of intracellular tyrosine kinases) responsible for transmitting growth and survival signals within the cell. If it is turned on, the cell grows or multiplies. Turning off this enzyme puts the cell into a state of rest. The hyperactive, uncontrolled mutant enzyme KIT triggers the rapid growth and endless proliferation of GIST tumor cells.
Understanding this process has already helped identify new treatments for this neoplasia.
Risk factors for GIST
Neurofibromatosis
The most significant risk factor for GIST is the presence of neurofibromatosis. It is almost always associated with the development of such neoplasms.
Family history
There are rare cases of hereditary GISTs. Hereditary tumors are characterized by inherited germline mutations in KIT or PDGFRA and additional syndromes such as:
- skin hyperpigmentation;
- irritable bowel syndrome;
- dysphagia;
- intestinal diverticulosis.
95% of GISTs are KIT positive. But 5% show no detectable KIT expression. In a subset of these KIT-negative GISTs, mutations occur in the PDGFRA gene rather than in KIT. PDGFRA immunostaining has been shown to help differentiate between KIT-negative GISTs and other gastrointestinal mesenchymal lesions.
BRAF and protein kinase Cθ (PKCtheta) mutations have also been reported in a small number of non-KIT/PDGFRA GISTs. Initial studies indicate that BRAF-mutant GISTs have a predilection for the small bowel and are not associated with a high risk of malignancy.
Current control
After complete tumor resection, ongoing clinical monitoring, including CT of the abdomen/pelvis and often CT of the chest (depending on the aggressiveness of the tumor), should be performed every 3-6 months for the first 5 years, and then once a year. For small tumors (<2 cm), longer intervals can be selected if necessary. It should be noted that with a long period of remission and with a low risk of relapse, an MRI of the abdominal cavity can also be performed as a control to reduce the level of radiation to the body. Ultrasound is not recommended for routine monitoring due to its inability to image peritoneal metastases. Gastroscopy as a follow-up type of control is currently no longer recommended, since the rate of local recurrence after complete tumor resection is very low.
As a control for metastatic GIST, a CT scan of the abdominal cavity and pelvis (to detect peritoneal and hepatic metastases) every 3-4 months is recommended. Typically, a chest CT scan is also recommended to rule out or detect pulmonary metastases.
Where is GIST treated in Germany?
Treatment of Russian-speaking patients with gastrointestinal stromal tumors is carried out according to the clinical recommendations of oncologists at the Nordwest Clinic. The hospital is equipped with high-tech equipment that allows you to quickly and accurately collect data for diagnosis. From the moment of treatment, the entire period of treatment and until the moment of discharge, each patient is accompanied by direct representatives of the clinic, managers and translators. Our employees will help you prepare the necessary documents and resolve any issues that arise along the way. Before coming to Nordwest Hospital, you can discuss your diagnosis with your doctor via video consultation.