- What causes bone pain?
- How are bone metastases diagnosed?
- How are bone pain treated?
Bone pain in cancer patients is usually caused by cancer cells that have invaded the bone - these are called bone metastases. Bone pain is often the first sign of bone metastases, so tests are carried out to confirm this diagnosis. Treatment of bone injuries is aimed at reducing pain, reducing the risk of fractures, treating fractures, and preventing or slowing the development of additional bone complications.
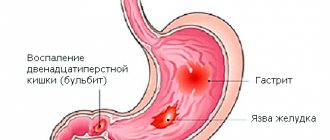
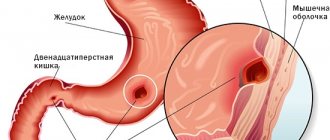
Metastases in the intestines: symptoms
Metastatic cancer means that the malignant process has reached the fourth stage. In this case, disturbances in the organ affected by the primary lesion become pronounced, the following are observed:
- pain that gradually intensifies;
- weakness and increased fatigue;
- loss of appetite and sudden weight loss;
- low-grade fever, which is not affected by taking antipyretics.
Our expert in this field:
Ivanov Anton Alexandrovich
Medical director, oncologist-surgeon, candidate of medical
sciences Call the doctor
Call the doctor
For cancerous tumors in the gastrointestinal tract:
- pain in the abdominal area;
- constipation, sensation of a foreign object during bowel movements;
- blood in the stool;
The same signs characterize the late stages of intestinal cancer with metastases. To establish an accurate diagnosis, detect all formations and determine further treatment strategies, modern research methods and analyzes are used: CT, MRI, ultrasound, tests for tumor markers, etc.
The lungs are the second most common target organ for colorectal cancer metastases after the liver. Approximately 20% of patients are diagnosed with distant metastases at the time of colon cancer diagnosis [1]. Of these, the proportion of patients with metastases in the lung (isolated lesion or in combination with damage to other systems and organs) is 10% [2]. In a significant number of patients with colorectal cancer, lung metastases are diagnosed metachronously at various times after treatment. According to some authors, in patients with rectal cancer, metastases in the lungs are more common than in patients with colon cancer [3].
Historically, metastases in the lungs in patients with colorectal cancer were considered as dissemination of the tumor process and any operation was considered pointless [4]. The development of drug antitumor therapy, surgical methods and analysis of treatment results with assessment of survival rates of patients with metastatic colorectal cancer made it possible to formulate the principles of a differentiated approach to treatment.
According to the developed protocols for the treatment of disseminated forms of colorectal cancer with multiple metastases in the lungs, multicomponent treatment is carried out. Much progress has been made in this direction. Thus, according to the recommendations of the NCCN (National Comprehensive Cancer Network), the following multicomponent drug programs are proposed, including cytostatic and targeted drugs: FOLFIRI or FOLFOX, or CapeOx ± bevacizumab; FOLFIRI or FOLFOX ± panitumumab or cetuximab (KRAS/NRAS only in wild type); FOLFIRI ± bevacizumab. Colon resection is performed if there is a risk of intestinal obstruction or bleeding. The effectiveness of drug treatment is assessed every 2 months from the perspective of possible resectability.
Despite the fact that the first report of lung resection for colorectal metastases was made by A. Blalock in 1944, the concept of surgical treatment began to take shape only in the 80s [5]. In 1979, Memorial Sloan-Kettering Cancer Center reported on 35 patients who underwent metastasectomy for colorectal cancer. This publication is considered by many scientists as a basic one, which gave rise to the study of the possibilities of the surgical method in the treatment of oligometastatic disease [6]. From 1986 to 1992, a number of scientific papers were published on the analysis of 10-year observations of patients after metastasectomy for colorectal cancer [7, 8]. In the 90s of the last century, an idea was formed about the validity of removing colorectal metastases from the lungs. Some authors have presented data on a twofold increase in the 5-year survival rate after metastasectomy (about 35%) [9, 10]. Since the 2000s, metastasectomy has gradually become part of clinical guidelines (NICE).
Currently, the most common view on the surgical stage of treatment of patients with metastatic lung disease: if the possibility of resection arises (primary tumor and metastases in the lungs), simultaneous or staged operations are performed. Then they offer three options: continuing intensive chemotherapy, observation, and short courses of chemotherapy.
According to the NCCN recommendations, in case of resectability of the primary tumor and metastases in the lungs, the first stage is to perform resection of the colon and lung simultaneously or sequentially, depending on the clinical situation. Next, adjuvant chemotherapy is carried out according to the FOLFOX or CapeOx regimens. The duration of drug treatment should not exceed 6 months.
It is also considered possible to begin treatment with neoadjuvant chemotherapy (FOLFIRI or FOLFOX, or CapeOx ± bevacizumab; FOLFIRI or FOLFOX ± panitumumab or cetuximab (KRAS/NRAS only in the wild type)) for a short course (2-3 months) followed by simultaneous or sequential operations. After surgical treatment, dynamic observation is carried out or a short course of adjuvant chemotherapy is carried out.
The third treatment option involves, at the first stage, performing a colon resection and chemotherapy (perioperative) according to the above regimens before surgery on the lung(s). After removal of metastases, it is possible to monitor the patient or conduct short courses of chemotherapy (for example, according to the CapeOx regimen).
The availability of drug treatment options indicates that there is no ideal drug regimen.
Survival
As can be seen from table. 1.5 year old
Table 1. Long-term results of treatment of patients after removal of colorectal cancer metastases from the lungs Note. * - after removal of a solitary metastasis, the overall 5-year survival rate was 33.4%, 10-year survival rate was 28.2%, and relapse-free survival rate was 31.4 and 20.4%, respectively. The survival rate of patients with colorectal cancer after surgical treatment of metastases in the lungs is 27-68%, and almost 40% of those operated on survive the 10-year mark. When analyzing these indicators, it is necessary to keep in mind that metastasectomies are more often performed in patients with a relatively more favorable prognosis for the course of the disease. In this regard, it is not entirely legitimate to recognize these indicators of long-term results of treatment of patients with generalized colon cancer as completely satisfactory.
Evidence-based medicine or a view from modern positions
Despite the prevailing opinion about the effectiveness of metastasectomy for colorectal cancer, not a single randomized study has been conducted to prove this judgment.
In light of the advances in drug treatment for patients with colorectal cancer and the changed regulations for the introduction of new treatment methods into practice, there is a need to rethink existing stereotypes. Thus, T. Åberg and T. Treasure [24] give their alternative interpretation of modern indicators of 5-year survival after metastasectomy. They believe that “good” 5-year survival rates (40–50%) are the result of patient selection and statistical illusion, for example, due to “lead time bias” and the selection of patients for surgical treatment [24, 25]. Surgeons, of course, operate on patients with solitary (one) and single (2-3) metastases, with a guarantee of radical resection, with the absence, according to diagnostic methods, of damage to the mediastinal lymph nodes. In addition, patients with so-called non-radical (R1/R2) operations are often not included in the analysis of long-term results [16, 26, 27].
Prognostic factors
When planning lung surgery, it is necessary to decide which patients can really benefit from this intervention. J. Pfannschmidt et al. [16] analyzed data from 17 published studies, including 1684 patients after surgical removal of colorectal cancer metastases. The five-year survival rate averaged 52.5%. Multivariate analysis revealed the following independent prognostic factors: the number of nodes, the size of the metastasis, the condition of the mediastinal lymph nodes, the interval of appearance of metastases after removal of the primary tumor, and the stage of the disease at the primary site before treatment.
Poor prognosis factors
Short period between removal of the primary tumor and the appearance of metastases in the lungs (DFI - dicease free interval)
M. Gonzalez et al. [22] analyzed 25 studies from 2001–2011, which included data on the treatment of 2925 patients with colorectal cancer with distant metastases (evidence level 1a). They showed that in 14 studies, short DFI was significantly (HR=1.59, 95% CI 1.27–1.98) a poor predictor of treatment.
According to some authors [28], short DFI is associated with another unfavorable factor—multiple metastatic lesions. P. Borasio et al. [19] believe that the best candidates for surgery are patients with solitary metastasis diagnosed 24 months after surgery at the primary site (colon), as this correlated with a better treatment prognosis (HR=0.478, 95% CI 0.282-0.811; R
=0,006).
Thus, the later the patient develops lung damage, the better the prognosis.
Number of metastases
Analyzing 20 publications of scientific studies on the treatment of patients with generalized colorectal cancer, M. Gonzalez et al. [22] showed that the number of metastases affects long-term treatment outcomes. The detection of single and multiple lesions is considered an unfavorable factor: HR=2.04, 95% CI 1.72-2.41. In many other retrospective studies, the authors also came to this conclusion [12, 13, 15, 19, 29].
Studying the effect on survival of the number of single lesions in the lungs, J. Cho et al. [23] showed that patients with multiple metastases (4 or more) had a significantly worse prognosis (HR=4.42, 95% CI 2.50–7.42; p
<0.001) than in those with solitary or single lesions (2-3 lesions). The authors came to the conclusion that it is advisable to surgically treat lung metastases when their number is less than three.
The presence of bilateral metastatic lung lesions in patients with colorectal cancer ( n
=84), according to F. Chen et al.
[17], is also an unfavorable prognostic factor: HR=5.06, 95% CI 1.04–24.64; p
=0.045.
In other words, the ideal model for surgery for pulmonary metastases in colorectal cancer is a patient with solitary metastases.
Metastasis size
In a study by H. Vogelsang et al. [14] revealed a worsening survival rate with a metastasis size of 3.75 cm or more (HR=2.6, 95% CI 1.4-4.9; p
=0,004).
Damage to the lymph nodes of the lung and mediastinum
The negative impact on the prognosis of surgical treatment of metastases in the lungs of colorectal cancer of factors such as damage to intrapulmonary and mediastinal lymph nodes has been shown in a number of studies [2, 11]. A large meta-analysis of 17 studies examining the prognostic significance of this factor showed its unfavorable effect on long-term treatment outcomes (HR=1.65, 95% CI 1.38-2.02) [22].
It should be emphasized that many studies have not achieved 5-year survival in the presence of metastases in the intrathoracic lymph nodes [30].
A more in-depth analysis of long-term results of treatment of patients with colorectal lung cancer with metastases in the lungs and lymph nodes suggested additional unfavorable prognostic factors, including the size of the metastasis more than 3 cm, the central location of the tumor and the multiple nature of the lesion [31-33]. J. Pfannschmidt et al. [27] showed in their work that damage to the lymph nodes of the root lobe of the lung is a predictor of damage to the lymph nodes of the mediastinum.
Apparently, neither the level of damage (bronchopulmonary or mediastinal) nor the number of affected lymph nodes correlates with survival; the fact of damage itself is important [20, 31]. Damage to the mediastinal lymph nodes reflects dissemination of the tumor process.
CEA (carcinoembryonic antigen) marker level
An elevated marker level as an independent factor of unfavorable prognosis has been established in many studies. A meta-analysis of 19 publications cited by M. Gonzalez et al. [22], showed that a high level of CEA marker before the thoracic stage of surgical treatment correlated with an unfavorable prognosis (HR=1.91, 95% CI 1.57-2.32).
Response to induction chemotherapy
Two studies over the past 5 years have shown improved survival rates in colorectal cancer patients after chemotherapy before metastasectomy ( p
=0,002—0,0001) [23, 34].
S. Cho et al. [29] presented data on a decrease in long-term treatment results when metastases appear during adjuvant chemotherapy: HR=1.982, 95% CI 1.083–3.631; R
=0,027.
The detection of new pulmonary metastases during induction therapy before the thoracic stage is also an unfavorable prognosis factor [32].
Other prognostic factors
Among other prognostic factors, researchers consider the patient’s age, primary localization of the tumor in the colon, radicality of metastasectomy, extent of lung resection (marginal resection or segmentectomy), etc. J. Cho et al. [23] showed a worse treatment prognosis in patients over 70 years of age: HR=1.95, 95% CI 1.25–2.47; R
=0,007.
In the work of S. Bölükbas et al. [32] the localization of the primary tumor in the rectum is considered as an unfavorable factor in the long-term results of treatment of patients: HR=2.93, 95% CI 1.55-5.55; R
=0.001. In this category of patients, a shorter time interval before the appearance of pulmonary metastases (DFI) becomes important in relation to patients with a colon tumor.
The detection of extrathoracic foci at the time of diagnosis of colon cancer, in addition to metastases in the lungs, is considered by some authors as an unfavorable factor in the prognosis of treatment ( p
=0,01—0,021) [13].
Oddly enough, at first glance, previous radical removal of liver metastases may not correlate with a poor prognosis. This should be taken into account when assessing the patient’s operability [22]. According to T. Mineo et al. [35], the 5-year survival rate in such patients was 51%. Favorable prognostic factors in this group should be considered young age, solitary liver damage, and long-term DFI [36].
Most researchers are unanimous in their opinion about the negative impact on long-term results of treatment of palliative operations on the lung, therefore they include only patients after radical interventions. The opposite opinion has been expressed by some authors in works of the last 5 years. Multivariate analysis proved the negative significance of non-radical metastasectomies R1 and R2: p
=0.0021 and
p
<0.0001 [21].
Some researchers include tumor doubling time less than 100 days, P53 mutation, the presence of clusters of tumor cells in the alveoli, the level of the cell adhesion protein E-cadherin, etc. as unfavorable clinical, morphological and immunohistochemical prognostic factors [37, 38].
Analysis of the results of surgical treatment of 112 patients with metastases of colorectal cancer in the lungs, operated on at the Moscow Oncology Research Institute named after. P.A. Herzen, confirmed the prognostic significance of the metastasis-free interval (DFI), the number of metastases in the lungs, the condition of the intrathoracic lymph nodes, the level of serological marker (CEA) and the nature of the operation performed. We consider DFI less than 36 months, the solitary nature of metastases in the lung, the absence of metastases in the intrathoracic lymph nodes, normal levels of CEA and R0 (radical) surgery to be favorable prognostic factors.
Thus, the search and analysis of prognostic factors is an important process towards the development and scientific substantiation of indications for the surgical stage of treatment of metastases in the lungs in patients with colorectal cancer.
Diagnostics
The scope of examination of patients with colorectal cancer includes computed tomography (CT) of the chest with contrast or positron emission tomography (PET) combined with CT (PET-CT), which are the main methods for diagnosing metastases in the lungs [39].
PET-CT is also informative in terms of assessing the condition of the mediastinal lymph nodes [20]. U. Pastorino et al. [39] showed 100% sensitivity of this diagnostic method in identifying affected mediastinal lymph nodes.
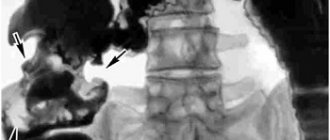
When intrapulmonary localization of metastases and planning thoracoscopic anatomical resection of the lung, in some cases it is advisable to perform 3D computer modeling, i.e., reconstruction of the vascular bed and bronchial tree of the affected lung, which makes it possible to clarify the topical location of the tumor (see figure)
CT scan of the chest (a) and 3D reconstruction of the lungs (b) of patient K., 63 years old. a — in the SVI of the left lung, a volumetric formation measuring 17x20 mm with tuberous spicule-like contours is visualized, surrounded by a zone of decreased pneumatization of the pulmonary parenchyma and with cords to the costal pleura; b — 3D reconstruction: 1 and 2 — vascular architecture, 3 — bronchial tree, 4 — neoplasm (metastasis) of the SVI segment of the left lung. [40, 41].
If it is necessary to differentiate a suspected lung metastasis from primary lung cancer or a non-tumor formation, it is possible to perform a fine-needle aspiration biopsy under CT navigation. The obtained material is sufficient not only for morphological examination, but also for immunohistochemical examination, which allows making a conclusion about the nature of the tumor [42].
Surgery
Surgery provides patients with solitary metastases of colorectal cancer in the lungs a chance of cure. Even after only resection of metastases without subsequent chemotherapy, many patients survive the 5-year mark. Observation of such patients created the basis for the concept of oligometastatic disease, justifying the possibility of using local methods of treatment for limited metastatic lesions [43].
Current NCCN guidelines explicitly recommend radical resection when lung metastases from colorectal cancer are identified [44]. In the NICE (UK) recommendations, experts are less categorical about the effectiveness of metastasectomy for lung lesions. British colleagues plan to get an answer to the question of the effectiveness of metastasectomy as a result of the currently ongoing randomized study PulMiCC [45, 46].
The use of a surgical method is considered from the perspective of the extent of resection (atypical, segmentectomy, lobectomy), the need for lymphadenectomy, and the choice of approach: thoracotomy or minimally invasive (VATS). In addition, it is necessary to compare the extent of surgical intervention with the functional state of the patient. In patients with low functional cardiorespiratory reserves, radiofrequency ablation or stereotactic radiotherapy (STRT) is considered. The best local control is achieved with small formations (5 cm is the limit of the method). Thus, O. Fanucchi et al. (2016), using radiofrequency ablation, provided 70.1% of patients with lung metastases with a diameter of less than 3 cm 5 —
summer local disease-free control.
In recent years, the possibilities of using SBRT for metastases in the lungs of tumors of various locations have been actively studied. However, the standard radiation dose and the number of fractions have not yet been unified. There are no studies comparing radiofrequency ablation and SBRT. Perhaps, over time, each method will occupy its own specific niche in the treatment of metastases.
Resection volume
Considering that the majority of metastases are localized in the mantle zone of the lung, it is often possible to limit the scope of surgery to atypical non-anatomical resection(s), ensuring sufficient distance from the tumor.
In a study by H. Vogelsang et al. [14] showed better survival of patients with colorectal cancer metastases in the lungs after non-anatomical resections compared to lobectomy or segmentectomy ( p
=0.006), which confirms the possibility of minimizing the volume of lung tissue resection without compromising the radicalism of the intervention.
Indications for pneumonectomy for metastases of colorectal cancer are very narrowed, on the one hand, due to its low oncological effectiveness: the 5-year survival rate of patients is only 16%, and on the other hand, due to the high risk of complications and mortality, which reaches 5%.
Select access
Minimally invasive thoracoscopic surgery has a number of advantages over open surgery regarding the rehabilitation of patients.
According to Y. Chao et al. (2012), a retrospective analysis of 35 statistically matched pairs did not reveal differences in overall survival ( p
=0.21) and relapse rate (
p
=0.23) in patients operated on through a thoracotomy or minimally invasive approach. However, to date, no randomized trial has been conducted to demonstrate the validity of VATS.
The main disadvantage of minimally invasive surgery is the limited ability to determine small nodes, foci located deep in the pulmonary parenchyma, and the true number of metastases (including X-ray negative), which is due to the lack of bimanual palpation. According to R. Ceroflio et al. (2009), 20% of metastases detected during thoracotomy were not diagnosed before surgery.
Considering the lack of differences in survival rates in the above studies and large meta-analyses between the thoracotomy approach and VATS, we can conclude that those formations that are not diagnosed and therefore not removed during minimally invasive surgery have little clinical significance. In addition, in a prospective study by J. Eckard et al. (2012) showed that the majority of formations identified as a result of bimanual palpation during thoracotomy access turned out to be benign.
The choice of surgical approach may be influenced by factors such as multiplicity of lesions, depth and size of the lesion. As can be seen from table. 2, most
Table 2. Dependence of the surgical approach on the characteristics of metastatic lesions of the lung (R. Ceroflio) surgeons often used VATS in patients with small (up to 1.5 cm) solitary metastases of subpleural localization.
We are proponents of the use of minimally invasive surgical technology and are confident that improvements in preoperative diagnostic capabilities and navigation systems will expand the indications for this technique.
Lymphadenectomy
Involvement of the mediastinal lymph nodes occurs in 10% of patients with colorectal metastases in the lungs [16]. However, the most correct would be to assess the condition of the lymphatic collector only in the group of patients after lobectomy, not including all atypical resections, since during the last volume of the operation there is no revision of the lymph nodes of the root lobe. In the work of S. Shiono et al. [31] the number of affected lymph nodes in patients who underwent lobectomy was slightly higher - 17% (58/344).
The need for mediastinal lymphadenectomy during operations for colorectal cancer metastases in the lungs is currently being actively discussed. As shown above, damage to this lymphatic collector is considered a factor of low survival, in other words, the presence of affected mediastinal lymph nodes indicates dissemination of the process. If this point of view is accepted, multiple biopsies of different groups of mediastinal lymph nodes will be sufficient for correct staging of the disease and planning of adjuvant chemotherapy [31].
At the same time, a number of authors are supporters of the principle of lymphadenectomy during metastasectomy. In a large study by T. Lida et al. [21], including 1030 patients, showed that the absence of lymphadenectomy was associated with a poor prognosis of the disease: HR=1.632, 95% CI 1.298–2.05, p
<0.0001. M. Hamaji et al. [20] based on their study also conclude that systematic mediastinal lymphadenectomy is necessary when removing lung metastases.
We advocate selective removal of lymph nodes, especially those that are clinically suspicious for disease.
Repeated resections
Approximately 30-50% of patients after metastasectomy are diagnosed with new metastases in the lungs during follow-up [8, 12]. It is believed that most of these patients, if the selection criteria are met, can undergo repeat surgery. The challenge is to identify the group of patients who will receive a clear survival benefit from repeat surgery.
In this regard, scientists from Fujita Heath University School of Medicine presented an interesting analysis. Of the 138 patients who underwent pulmonary metastasectomy, 35 were subsequently diagnosed with recurrent pulmonary metastases, and 33 of them were reoperated. Thus, in the group of primary metastasectomies, reoperations amounted to 24% (33/138). The time interval between the first and reoperation averaged 12 months (5–37 months). The average number of metastases diagnosed again was 2 (1–6). Patients more often underwent atypical resection (in 20), less often - segmentectomy and lobectomy (in 5 and 7 patients, respectively). Pneumonectomy was required in 1 patient. It should be noted that in almost all patients, the primary operation was performed using the VATS method - 31 (94%) out of 33. In addition, in more than half of the patients it was possible to perform a reoperation also in a minimally invasive version - 21 (68%) out of 31. Repeated operations are accompanied by a low incidence of complications. The five-year survival rate of 33 reoperated patients was 64.3%: 27.3% were alive without signs of relapse and 27.3% were alive with signs of relapse. The survival rate of those operated on again does not differ from that in the group of patients who did not have a second relapse in the lungs - 105 (61.3%) patients versus 33 (64.3%), respectively.
Similar data are provided by D. Nachira et al. (2016) from Catholic University (Italy). Long-term results in the group of patients with repeated lung operations do not differ significantly from those in the absence of recurrent metastases: 56 and 58% ( p
=0.182) respectively.
Some authors consider an increased level of the tumor marker CEA to be an unfavorable prognosis factor for the treatment of patients with repeated operations for recurrent metastases in the lungs. Five-year survival rate with elevated CEA levels is 47%, and with normal levels it is 90% ( p
=0,002).
D. Nachira et al. (2016) found a trend toward improved survival in patients who did not undergo liver resection before the pulmonary stage: 5-year survival rate 85% versus 34% ( p
=0.008).
In the group of patients who underwent liver resection, better 5-year survival was with unilateral lung lesions than with bilateral ones (52 and 23%, respectively, p
= 0.014), as well as with solitary metastases than with multiple ones: 2-year survival was 67 and 25%, respectively (
p
= 0.004).
Thus, today it is justified to plan repeated operations for metastases of colorectal cancer, but subject to careful selection of patients.
Monitoring
Monitoring programs are generally similar in all countries. The examination is performed every 3-6 months for 2 years, then every 6 months for 5 years. CEA levels are determined every 3-6 months for 2 years, then every 6 months for 3-5 years. CT (chest, abdomen, pelvis) - every 3-6 months for 2 years, then every 6-12 months up to 5 years [44, 46].
Despite progress in the treatment of colorectal cancer patients with pulmonary metastases, the effectiveness of metastasectomy, assessing the margin of effectiveness of surgery, and determining the group of patients for whom removal of pulmonary metastases will be useful remain unresolved.
To date, surgery remains the main stage in the treatment of colon cancer with metastases to the lungs. The proven effectiveness of drug therapy requires a combination of these two methods. The presence of options in the construction of treatment programs means that there is no clear advantage of one of the protocols. Analysis of prognostic factors creates the basis for a differentiated approach to determining indications for removal of pulmonary metastases. In this regard, it is recommended to take into account the size of metastases, their number, damage to the mediastinal lymph nodes, the level of CEA and the response to induction chemotherapy.
The authors declare no conflict of interest.
Information about authors
Ryabov Andrey Borisovich
- https://orcid.org/0000-0002-1037-2364; e-mail
Book your room today
6 seats
1 local ward
- 4 meals a day
- Bathroom in the room
- Anti-decubitus mattresses
- Nurse
6700 rub.
Book
13 places
2 local ward
- 4 meals a day
- Bathroom in the room
- Anti-decubitus mattresses
- Nurse
4200 rub.
Book
2 seats
VIP chamber
- a guest room
- 4 meals a day
- Bathroom in the room
- Anti-decubitus mattresses
- Nurse
16200 rub.
Book
6 seats
1 local ward
- 4 meals a day
- Bathroom in the room
- Anti-decubitus mattresses
- Nurse
6700 rub.
Book
13 places
2 local ward
- 4 meals a day
- Bathroom in the room
- Anti-decubitus mattresses
- Nurse
4200 rub.
Book
2 seats
VIP chamber
- a guest room
- 4 meals a day
- Bathroom in the room
- Anti-decubitus mattresses
- Nurse
16200 rub.
Book
Bisphosphonates
A group of medications called bisphosphonates can effectively reduce bone loss that occurs from metastatic lesions, reduce the risk of fractures, and reduce pain. Bisphosphonates act by inhibiting bone resorption or destruction. Bone tissue is continuously exposed to two types of cells: osteoclasts, which destroy old bone cells, and osteoblasts, which restore it. In turn, cancer cells secrete various factors that stimulate osteoclast activity. Although the exact mechanism of action of bisphosphonates is not entirely clear, they are thought to inhibit and destroy cell-destructive osteoclasts, thereby reducing bone breakdown. Data from more than 30 clinical studies show that patients with bone metastases who were treated with bisphosphonates were less likely to have fractures, less likely to need radiation therapy, and less likely to have hypercalcemia (increased levels of calcium in the blood). Bisphosphonates have been shown in clinical studies to prevent or slow bone change and associated pain in patients. Most often, bone metastases occur with:
- Breast cancer
- Prostate cancer
- Lungs' cancer
- Myeloma
- Kidney carcinoma
Reviews
23.04.2021
“I was surrounded by care, love and relieved of pain!”
16.04.2021
Feedback from a patient at the NACFF clinic after surgery
14.04.2021
Mass in the transverse colon
05.03.2021
Colon and rectosigmoid cancer
View all reviews
Hospital, hospitalization, emergency medical care - 24/7, 7 days a week
+7 (495) 259-44-44
What causes bone pain?
A common cause of bone pain is metastatic cancer. The spread of cancer from its original location to another part of the body is called metastasis. Bone metastases are not new or different cancers—they are made up of cancer cells from the primary cancer, such as breast, prostate, lung, kidney, or thyroid cancer cells, that have spread to the bone.
Cancer cells can spread, i.e. metastasize throughout the body and into the lymphatic system. Bones are one of the most common sites in the body to which cancer metastasizes. Bone metastases usually enter the bloodstream. Cancer cells detach from their original location and travel through blood vessels until they attach to the vessel wall of a small capillary network in bone tissue. Cancer can also spread to bone by direct growth from a nearby tumor, although this is much less common than spread through the bloodstream.
Pain in bone cancer occurs because the cancer disrupts the normal balance of cells in the bones, causing changes in the structure of the bone tissue. In healthy bone, there is a constant process of remodeling, i.e. destruction and restoration of bone tissue occurs. Cancer cells that have spread to the bone upset this balance between the work of osteoclasts (cells that break down bone) and osteoblasts (cells that form new bone), causing either less or increased bone formation. These disorders can either affect the periosteum (the tough membrane covering the bone, also called the bony film ) or stimulate the nerves in the bone, causing pain.