A pancreatic cyst is a cavity that is surrounded by a capsule and filled with fluid. The most common morphological form of cystic lesions of the pancreas are postnecrotic cysts. At the Yusupov Hospital, doctors identify cysts in the pancreas through the use of modern instrumental diagnostic methods: ultrasound, retrograde cholangiopancreatography, magnetic resonance imaging (MRI), computed tomography (CT). Patients are examined using the latest diagnostic equipment from leading global manufacturers.
The increase in the number of patients with cystic lesions of the pancreas is facilitated by the indomitable increase in the incidence of acute and chronic pancreatitis, the increase in the number of destructive and complicated forms of the disease. The frequency of postnecrotic pancreatic cysts is increasing due to the introduction of effective methods of conservative treatment of acute and chronic pancreatitis.
Against the backdrop of intensive therapy, therapists at the Yusupov Hospital are increasingly able to stop the process of destruction and reduce the frequency of purulent-septic complications. Surgeons use innovative techniques for treating pancreatic cysts. Severe cases of the disease are discussed at a meeting of the Expert Council with the participation of professors and doctors of the highest category. Leading surgeons collectively decide on patient management tactics.
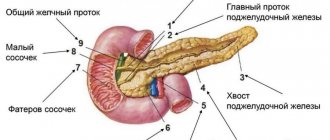
Where is the organ located and what parts does it consist of?
The gland is located in the area behind the peritoneum, to the left and slightly behind the stomach. It is protected by ribs and consists of a head, tail and body, so scanning of the sections occurs from different points. At the heart of the organ are small lobules that produce digestive enzymes and pancreatic islets that release humoral substances into the blood. Pancreatic juice, which serves as an enzyme, enters the duodenum and takes part in the digestion process.
Preparatory activities
The scan should be done on an empty stomach. To do this, the patient must fulfill several requirements:
• 24 hours before the procedure, exclude “heavy foods” from the diet.
• Dinner should be no later than 18:00.
• To relieve bloating and reduce gas formation, you can take Espumisan.
You need to bring a diaper and a towel to the ultrasound scan. During the examination, the patient lies on the couch. The ideal option would be to perform an ultrasound in the morning. In some cases, the patient will need to drink water before the procedure.
How is an MRI of the pancreas done?
The greatest diagnostic value is for images obtained on high-field units with a closed contour
Before scanning, you must leave items containing metal in the storage compartment. A cell phone or payment card forgotten in a pocket when exposed to a magnetic field causes distortion in the photo.
After completing the documentation, the x-ray technician escorts the patient to the diagnostic room and places him on the scanner table. The limbs are fixed with soft belts and bolsters, and an intensifying coil is placed above the abdomen.
If an MRI of the pancreas with contrast is planned, a catheter is inserted into the vein. During certain phases of the study, a chelated gadolinium enhancer will be automatically delivered through the drain (using an injector) to improve visualization.
Medical staff monitors the progress of the diagnosis through glass from an adjacent room. Communication takes place via speakerphone; in case of an unforeseen event (an attack of claustrophobia, deterioration in health), the patient has a special signal button at hand. Technical noise produced by equipment can be leveled out using headphones.
After the native series of images, there is a wait for the dye to spread through the bloodstream to the organs of the biliary tract. The duration of MRI with contrast is 30-45 minutes. The results will be ready within an hour. There are no obstacles to daily activities.
How is the research going?
First, the patient lies on his back, then he turns on his left side. In this position, the tail of the gland is scanned. After this, he turns on his right side. To examine the condition of the head and body of the gland, the doctor asks the patient to take a semi-sitting position.
As landmarks for detecting the pancreas, the sonologist uses the aorta with the inferior vena cava, superior and inferior mesenteric arteries, etc. There is a special program to determine the size of the organ. Even if all the indicators do not deviate from the norm, the doctor provides a detailed breakdown of the data in the conclusion.
The density of pancreatic tissue (echogenicity) depends on the age and body weight of the patient. Over the years, it decreases and acquires signs of hyperechogenicity. Normally, the structure of the gland is homogeneous. Proper preparation facilitates good viewing of all parts of the organ during the examination.
A case of ultrasound diagnosis of a non-functioning neuroendocrine tumor of the pancreas
Ultrasound scanner HS50
Affordable efficiency.
A versatile ultrasound scanner with compact design and innovative capabilities.
Introduction
Neuroendocrine tumors (NETs) are neoplasms capable of synthesizing biologically active substances, the source of which are cells of the diffuse endocrine system (DES). The latter can deposit precursors of biogenic amines and synthesize them along with polypeptide hormones. Genetically, DES cells belong to the cells of the APUD system (amine precursor uptake and decarboxilation), therefore, for a long time, tumors from these cells were called “apudomas”. In the domestic literature, pancreatic NETs are often called carcinoids [1], while in foreign literature, carcinoids mean only “true” serotonin-producing tumors [2].
NETs of the digestive organs, according to various statistical data, are detected with a frequency of 12-15 per 1 million population, and among them, pancreatic neoplasms account for up to 70-80% [3, 4]. NETs of the pancreas, along with tumors of the lungs, bronchi, gastrointestinal tract, kidneys, and skin, account for less than 1% of all human malignancies [5, 6] and even large clinics rarely have experience in treating more than 100 such patients [7].
There are functioning and non-functioning NETs. In the majority of observations (75-80%), patients with NETs with clinical symptoms may develop severe pathological conditions caused by the hormonal activity of these tumors, such as hypoglycemia, Zollinger-Ellison syndrome, Werner-Morrison syndrome, etc. In addition, some of these syndromes can be caused not only by a neoplasm of the pancreas, but also by NETs of other localization, primarily the stomach and duodenum, as well as microadenomatosis with nesidioblastosis. As a rule, if there is a clinical picture of the disease, making a syndromic diagnosis in patients with NET is not very difficult. The situation is more difficult with the diagnosis of NETs in the absence of their activity or with so-called non-functioning tumors.
Thus, in 30-35% of cases, the development of NETs is not accompanied by the development of hyperfunctional syndromes, therefore such tumors are often called non-functioning [8]. The term “non-functioning” NETs is very arbitrary and reflects only the absence of specific symptoms and syndromes in the clinical picture of the disease. In this case, tumor cells can either produce a functionally inert hormone, or produce it in quantities insufficient for clinical manifestation, or produce a hormone that does not cause specific symptoms, or the mechanism for implementing the action of hormones may be disrupted. Such tumors often include PP-cell, A- and D-cell tumors [8, 9]. The clinical picture of non-functioning pancreatic tumors consists of nonspecific symptoms, often caused by compression of surrounding organs and tissues, less pronounced than with other malignant neoplasms of the gland. The general condition of patients remains relatively satisfactory for a long time [7, 8, 10]. Hormonal hyperfunction syndromes can manifest themselves only in late stages, when the tumor reaches a large size or distant metastases appear. Therefore, the size of detected tumors averages 10 cm (from 3.5 to 20 cm) [11-13]. The anatomical connection of the head of the pancreas with the common bile duct and duodenum and their relatively rapid involvement in the pathological process determine that the majority (54%) of non-functioning NETs of the pancreas are diagnosed in its head [13].
NETs of the gastrointestinal tract are characterized by a long course and metastasis more rarely than typical adenocarcinomas. Typically, well-differentiated tumors with a diameter of up to 2 cm are benign; tumors larger than 3 cm are classified as either tumors with borderline malignant potential or malignant. The frequency of metastasis of carcinoid tumors correlates with the degree of differentiation and size of the tumor. With a primary tumor less than 1 cm in diameter, metastases are detected only in 2% of cases, with a tumor whose size exceeds 2 cm - in 90% of patients [14].
The main role in making a diagnosis, determining the extent of the tumor process and deciding on treatment tactics belongs to instrumental examination methods.
We have not come across any descriptions of cases of successful ultrasound diagnosis of a non-functioning neuroendocrine tumor of the pancreas in its caudal region in the literature. That is why in this message we want to share our experience.
Clinical observation
Patient K., 51 years old, underwent a screening ultrasound of the abdominal organs for the first time in his life. For several years he has been experiencing weight gain and periodic discomfort in the epigastric zone. There were no complaints at the time of the examination. The ultrasound was performed on an expert-class digital ultrasound scanner Accuvix-XQ (Medison). The size of the liver is moderately enlarged. The parenchyma of the liver and pancreas has increased echogenicity, the bile ducts and the pancreatic duct are not dilated. Gallbladder, spleen, kidneys - no signs of organic pathology. In the upper quadrant of the abdominal cavity, in the area “caudal pancreas - upper pole of the left kidney - hilum of the spleen”, an additional formation of irregular round shape, reduced echogenicity, with fairly clear, uneven contours, with dorsal pseudo-amplification of the echo signal, size 3, was detected. 0x2.8x2.7 cm (volume 11.8 ml). With CDK, the formation was defined as hypovascular. Extravasal compression of the great vessels, signs of their germination, as well as signs of diffuse transition to nearby tissues were not detected. The formation was better visualized in orthostasis (standing). Conclusion: Additional formation in the upper quadrant of the abdominal cavity of unknown organ origin, diffuse changes in the parenchyma of the liver and pancreas (Fig. 1).
Rice. 1.
An additional round hypoechoic formation in the upper outer quadrant of the abdominal cavity in the projection of the hilum of the spleen.
For the purpose of topical diagnosis of the formation, computed tomography (CT) was performed on a 32-slice computed tomograph. On tomograms, the liver is enlarged due to the right lobe, with clear and even contours, its structure is homogeneous, the intrahepatic bile ducts are not dilated. Densitometric parameters of the parenchyma are reduced to 43-46 HU. No focal formations were found in the liver. The portal and splenic veins are not dilated. The common bile duct is of normal width - 0.45 cm. The gall bladder is of normal size, its contents are homogeneous. No radiopaque stones were detected. The shape and dimensions of the head and body of the pancreas are not changed (head thickness - 2.9 cm; body thickness - 3.6 cm), densitometric parameters of the parenchyma are not changed, lobulation is preserved. At the level of the tail, the lobulation is smoothed, its thickness is up to 3.6 cm. In the structure of the parenchyma of the gland at this level, an additional rounded formation is detected, measuring 3.3 (vertical) x 3.7 (transverse) x 4.1 (antero-posterior) cm ( volume 26.0 ml). The formation is adjacent to the outer contour of the left kidney at the level of the border of the upper and middle third. In the native phase, the density of the formation is isodense to the pancreatic tissue (33-35 HU); in the arterial phase, the formation tissue actively accumulates the contrast agent, and an enhancement of up to 74 HU is observed. Its contours are clear, its structure is homogeneous. In the parenchymal phase, the density of the formation is isodense to the gland tissue; in the delayed phase, the symptom of “contrast washout” is observed, the density of the formation is up to 52 HU; A clear capsule up to 0.4 cm is contoured. The Wirsung duct is not dilated. Peripancreatic fiber is homogeneous. The spleen is not enlarged, uniform in density. Metastases to the lymph nodes and liver were not detected. Conclusion: CT signs of additional hypervascular formation of the tail of the pancreas, moderate hepatomegaly, dystrophic changes in the liver parenchyma like fatty hepatosis (Fig. 2).
Rice. 2.
MRI of an additional mass in the caudal pancreas (arrows).
A)
Native image, the tissue of the formation is isodense to the tissue of the pancreas.
b)
Active accumulation of contrast agent by the tissue of the formation, mostly in the periphery.
During laboratory diagnostics, the level of carbohydrate antigen in the blood (marker of pancreatic carcinoma - CA-19-9) was 10 U/ml (with reference values less than 37).
During the consultation, the surgeon suggested that the patient had a non-functioning neuroendocrine tumor of the pancreas and suggested its surgical removal. Due to the lack of complaints, the patient categorically refused the operation.
After 2 months, with repeated ultrasound, the size of the tumor did not change significantly (Fig. 3). After an explanatory conversation, the patient consented to surgical treatment.
Rice. 3.
An additional round hypoechoic formation in the upper outer quadrant of the abdominal cavity in the projection of the hilum of the spleen in two mutually perpendicular projections without signs of dynamics.
In the surgical department upon examination: the condition is satisfactory. Normal physique, skin and visible mucous membranes of normal color. In the lungs there is vesicular breathing, no wheezing. Heart sounds are rhythmic, no murmurs are heard. Pulse 80 beats per minute, satisfactory filling, blood pressure 130/90 mm Hg. Art. The abdomen is soft and painless on palpation in all parts. Pasternatsky's sign is negative on both sides, urination is not impaired. Esophagogastroduodenoscopy revealed erythematous (focal) gastropathy. Laboratory tests (biochemical blood test, coagulogram determination, clinical blood test, general urine test) did not reveal diagnostically significant abnormalities.
The choice of surgical treatment method was determined by the location and size of the tumor. A distal pancreatectomy with splenectomy was performed.
The material represented by the resected part of the pancreas (9.0 cm long) and the spleen (10.0×6.5×4.0 cm) was sent to the pathology department for examination as a single block. When studying the macroscopic specimen: the pancreatic capsule is not tense, its tissue on sections is gray-yellow in color, lobulated. At 5.5 cm from the edge of the resection of the gland and 2.0 cm from the spleen, a clearly defined node in the capsule (5.0x6.0x4.5 cm, volume 70.2 ml) of a soft-elastic consistency, yellow-brown color is determined on sections, relatively uniform. The resection margin of the gland is of normal structure, without signs of tumor growth. The spleen is bluish-gray, elastic, its capsule is thin, the pulp is dark cherry in color, and gives scanty scraping.
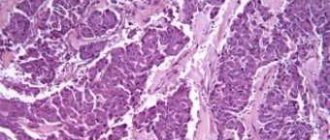
Histological examination confirmed the neuroendocrine nature of the formation. Microscopically, the node in the pancreas is represented by tumor tissue, built from medium-sized and large cells with moderate nuclear polymorphism, a rather extensive rim of eosinophilic and optically empty cytoplasm, forming solid, alveolar, trabecular, pseudoglandular and glandular structures, in some preparations - with perivascular orientation, with a large number of capillary and sinusoidal vessels (Fig. 4). Mitotic activity is very low (0-1 mitosis in 10 representative fields of view). Grimelius staining reveals fine granularity in the cytoplasm of tumor cells. The fibrous capsule of the tumor is quite wide, no signs of invasion of tumor tissue into the capsule and vessels were detected, and no necrosis was detected. In the pancreatic tissue surrounding the tumor there are dystrophic changes and interstitial edema. No tumor growth was detected at the pancreatic resection margin. In the parapancreatic tissue there are focal hemorrhages and mild focal lymphocytic infiltration. In the spleen there is uneven blood supply to the red pulp, sclerosis of arterioles.
Rice. 4.
Pathohistological picture of pancreatic tumor cells. The cells form glandular and trabecular structures with a perivascular orientation. Hematoxylin and eosin staining, x200.
To verify the neuroendocrine nature of the tumor, broad-spectrum immunohistochemical markers were used: antibodies to chromogranin A and synaptophysin, as well as antibodies to hormones (insulin, glucagon, somatostatin, gastrin, pancreatic polypeptide, serotonin, calcitonin, adrenocorticotropic hormone). To detect ductal differentiation of the tumor, antibodies to broad-spectrum cytokeratins and selectively to cytokeratin 19, carcinoembryonic antigen, epithelial membrane antigen and p53 protein were used (table).
Table
. Immunohistochemical study.
| Antibody name | Result |
| CD 56 (Clone SPM 128, Spring Bioscience) | + |
| CD 34 (Clone QBEnd 10, Dako) | — |
| Vimentin (Clone V9, Dako) | — |
| Chromogranin A (Clone LK2H10, BioGenex) | -/+ |
| S 100 (Polyclonal Rabbit Anti-Cow, Dako) | + |
| NSE (Polyclonal Antibody, Spring Bioscience) | + |
| Cytokeratin (Clone MM-1 16, Dako) | +/- |
| Synaptophysin (Clone Snp 88, BioGenex) | -/+ |
| Cytokeratin 8 (Clone C 51, BioGenex) | + |
| EMA (Clone E 29, Diagnostic BioSystems) | -/+ |
| Ki-67 (Clone MIB-1, Dako) | positive cells 0-1 in 10 representative fields of view |
Tumor cells expressed CD 56 (Clone SPM 128, Spring Bioscience), S 100 (Polyclonal Rabbit Anti-Cow, Dako) (Fig. 5), NSE (Polyclonal Antibody, Spring Bioscience), Cytokeratin 8 (Clone C 51, BioGenex). About half of the cells expressed pancytokeratin. Chromogranin A (Clone LK2H10, BioGenex) and Synaptophysin (Clone Snp 88, BioGenex) were expressed in single tumor cells. A marker of proliferative activity (Ki-67, Clone MIB-1, Dako) was expressed in the nuclei of 0-1 cells in 10 representative fields of view. Antibody to CD 34 (Clone QBEnd 10, Dako) revealed a network of capillary and sinusoidal vessels.
Rice. 5.
Immunohistochemical study of a removed pancreatic tumor. Tumor cells express S-100. x400.
Pathohistological conclusion: The histological structure of the tumor and the immunophenotype of tumor cells (taking into account tumor size and low mitotic activity) correspond to a non-functioning neuroendocrine tumor of the pancreas with uncertain malignant potential.
The postoperative period proceeded without complications. On the 24th day, the patient was discharged in satisfactory condition under the supervision of an oncologist.
Discussion
There is no doubt that clinically “silent” non-functioning NETs of the pancreas, located in the tail of the organ, represent a very difficult group of diseases for primary diagnosis.
However, the above observation shows that a standard ultrasound examination performed in the screening mode makes it possible to detect such tumors precisely at the stage of “clinical well-being.” Thus, we identified an asymptomatic “accidental finding” during the first ultrasound in an adult patient’s life, located in the upper outer quadrant of the abdominal cavity, and turned out to be a non-functioning neuroendocrine tumor of the pancreas with uncertain malignant potential. At the same time, the ultrasound results served as the basis for further rational, multifaceted diagnostic examination of the patient, which made it possible to timely and effectively carry out a technically complex operation to remove the tumor at an early preclinical stage.
Note that the size of the tumor on ultrasound was 16% of the true (pathomorphological) size, which may likely indicate the resulting effect of refraction of ultrasonic waves, leading to geometric distortions of the resulting image [15]. We assume that this fact has important diagnostic and prognostic significance for tumors in the described area.
Expanding preventive measures to identify diseases of the pancreas, increasing the responsibility of doctors not only in ultrasound diagnostics, but also in other specialties, as well as the attention of patients to their health, will only help improve the diagnosis and prognosis of non-functioning NETs of the pancreas, and a more detailed assessment of ultrasound data will allow use the method more widely in diagnostics at the preclinical stage of development of this pathology.
conclusions
- Non-functioning NETs located in the tail of the pancreas are rare forms of neoplasms and, as a rule, are characterized by slow growth and an asymptomatic course until they begin to compress surrounding organs and tissues during growth or when metastases appear.
- When an additional round-shaped formation in the upper outer quadrant of the abdominal cavity of a homogeneous structure of reduced echogenicity, up to 3 cm in size, is identified during ultrasound, it is advisable to supplement it with computed tomography.
- The size of non-functioning NETs detected by ultrasound may be less than 20% of the true ones, due to the physical effect of refraction of the ultrasound beam.
Literature
- Savvina T.V. Histotopographic study of the pancreas // Arch. Pat. 1979. N11. pp. 68-73.
- Creutzfeldt W. Carcinoid Tumors: Development of Our Knowledge // Wld J Surg 1996. V. 20. P. 126-131.
- Eriksson B., Arnberg H., Lindgren PG et al. Neuroendocrine pancreatic tumors: clinical presentation, biochemical and histopathological findings in 84 patients // J. Int. Med. 1990. V. 228. P. 103-113.
- Gibril F., Jensen RT Somatostatin receptor scintigraphy in the Zollinger — Ellison syndrome // Ann. Int. Med. 1997. V. 126. P. 741-742.
- Benjegard SA, Flesher N, Allan RN, Siperstein E. Laparoscopic Radiofrequency Ablation of Neuroendocrine Liver Metastases // Wld J Surg. 2002. V. 26. P. 985-990.
- Capella C., Heitz P.U., Hofler H. et al. Revised Classification of Neuroendocrine tumors of the lung, pancreas and gut // Digestion 1994. V. 55. Suppl 3: P. 11-23.
- Bax NDS Woods HF, Batchelor A., Jennings M. Clinical manifestations of carcinoid disease // Wld J Surg. 1996. V. 20. P. 142-146.
- Norton JA, Kivlen M, Li M et al. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors // Arch. Surg. 1999. V. 138. N8. P. 859-866.
- Abdulkerimov Z.A. “Non-functioning” neuroendocrine tumors of the pancreas: Diss. ...cand. med. sc. M., 2001.
- Kuzin N.M., Egorov A.V. Neuroendocrine tumors of the pancreas. M: Medicine, 2001.
- Woods HF, Batchelor A., Jennings M. Clinical manifestations of carcinoid disease. Wld J Surg. 1996. V. 20: R. 142-146.
- Kuzin N.M., Egorov A.V., Kondrashin S.A. et al. Diagnosis and treatment of gastrin-producing tumors of the pancreas // Klin. honey. 2002. N 3. P. 71-76.
- Grant CS Gastrointestinal endocrine tumors. Insulinoma. Baillieres // Clin. Gastroenterol. 1996. V. 10. N4. P. 645-671.
- Kim DG, Chejfec G., Prinz RA Islet-cell carcinoma of the pancreas // Am Surgeon 1984. V. 55. R. 325.
- Osipov L.V. Ultrasound diagnostic systems: physical principles and methods. Practical guide to ultrasound diagnostics. General ultrasound diagnostics / Ed. Mitkova V.V. M.: Publishing house Vidar-M. 2003. P. 10. pathology.
Ultrasound scanner HS50
Affordable efficiency.
A versatile ultrasound scanner with compact design and innovative capabilities.
Pathological changes
- An abscess (abscess) of the pancreas looks like a pocket or bag filled with sequesters and heterogeneous fluid. As the patient's body position changes, the level of fullness changes.
- Acute or chronic pancreatitis, causing changes in the structure of a diffuse or focal nature. At the same time, the tissue density is reduced, the contours of the organ are not clear. The size of the gland and its ducts is higher than normal. Based on these changes, the doctor diagnoses pancreatitis.
- The appearance of necrotic foci and cysts further provokes the complete melting of pancreatic tissue (pancreatic necrosis). This appears as poorly structured hypoechoic areas.
- One of the consequences of pancreatic necrosis is the formation of many purulent abscesses, which, merging, form large areas of pus and sequestration.
- False cystic formations on the screen look like anechoic cavities filled with fluid.
- Tumors on ultrasound look like round or oval formations of low density and heterogeneous structure, abundantly covered with a network of blood vessels. If cancer is suspected, all parts of the gland must be examined very carefully. If the focus of a malignant tumor is in the head, then the sign will be jaundice. Ultrasound data also allows you to diagnose the type of cancer.
MRI of the pancreas: indications
What is better, to do a magnetic resonance or computed tomography, the doctor decides, taking into account the clinical situation and restrictions on the use of the method
There are many indications for MRI of the pancreas. It is recommended to sign up for the procedure if:
- pain in the epigastric region, abdomen, indigestion, inexplicable by tests and ultrasound, appeared, indigestion - foul-smelling stool, unusual color of stool, nausea, vomiting, flatulence, jaundice, etc.;
- family history is burdened - several first-degree relatives have been diagnosed with pancreatic cancer;
- preoperative diagnostics are necessary to study the anatomical features and plan the intervention;
- ultrasound results are ambiguous/suspicious for pancreatic pathology, which implies advanced diagnostics;
- differentiation between cancer and chronic pancreatitis is required;
- deviations from the norm in laboratory parameters indicate disease of the pancreas, gallbladder, ducts, liver;
- there is a suspicion of a tumor in the biliary system, it is necessary to determine the stage of the oncological process, relapse after treatment;
- there are contraindications to computed tomography, including enhanced CT (pregnancy, childhood, allergy to iodine-containing contrast, renal failure, hyperfunction of the thyroid gland);
- There is no effect of therapy for pancreatitis / frequent exacerbations are observed when following a diet (MRI of the pancreas shows choledocholithiasis, intraluminal stones, stage of inflammation);
- it is necessary to exclude postoperative complications, etc.
Calcifications in the pancreas on ultrasound
Important!!! If there is dilatation of the pancreatic duct, you should look for stones in the pancreatic duct and in the common bile duct.
Calcifications inside the pancreas can give an acoustic shadow, but if they are small, they appear as a separate bright echostructure without an acoustic shadow. In chronic pancreatitis, calcifications are distributed diffusely throughout the pancreas. Stones in the duct are located along the duct. Gallstones in the distal common bile duct may be mistaken for calcifications in the pancreas. Calcifications are clearly visible on CT, and for non-calcified stones, MRI or ultrasound is preferable.
| Photo. A - There is a small stone in the dilated duct. B - In the dilated pancreatic duct there is a row of several stones with shadowing behind. B — A patient with chronic pancreatitis has huge stones in the dilated duct. Note the intense shading behind. | ||
| Photo. A, B — Calcifications in the pancreatic parenchyma in patients with chronic pancreatitis. Some calcifications have a shadow. B — A 5-year-old boy with chronic hereditary pancreatitis: calcifications (small arrows) and dilatation of the pancreatic duct (large arrow). C - confluence of the superior mesenteric and splenic veins. | ||
Acute pancreatitis on ultrasound
Acute pancreatitis is a severe complication of gallstone disease or a consequence of toxic effects, such as alcohol.
Mild pancreatitis is not visible on ultrasound (CT is a more sensitive method). Severe pancreatitis is easily detected by ultrasound. When an unusually clear and contrasting pancreas stands out from the surrounding tissue, edema of the parenchyma and surrounding fatty tissue can be assumed. If a thin layer of free fluid is visible around the pancreas, along the stomach, at the hilum of the liver and spleen, pancreatitis can be confidently diagnosed.
| Photo. Acute pancreatitis on ultrasound: A - Swelling of the pancreatic parenchyma (p), the contour of the pancreas is unusually clear, a small accumulation of fluid along the border (arrows). B, C - Accumulation of fluid along the contour of the body of the pancreas, a thin rim of fluid along the splenic vein (arrows), the parenchyma is heterogeneous, the surrounding tissue is hyperechoic - swelling and inflammation, the common bile duct is dilated (B). In this case, gallstone disease must be excluded. | ||
Almost all pancreatic tumors are hypoechoic compared to the normal pancreas. It is impossible to distinguish between focal pancreatitis and pancreatic tumor using ultrasound alone. A tumor and pancreatitis can be combined.
| Photo. Acute pancreatitis on ultrasound: The pancreas is unusually contrasted against the background of hyperechoic surrounding tissues, a thin strip of fluid along the contour (A), a hypoechoic focus in the tail (B), fluid in the hilum of the spleen (C). A hypoechoic tail can be mistaken for a tumor. | ||
In severe cases of pancreatitis, pancreatic fluid digests surrounding tissue, forming pseudocysts. Such cysts can be single or multiple. They may increase in size and rupture.
On ultrasound, pseudocysts are defined as oval or round hypoechoic formations with clear contours. In the early phases of cyst formation, it is a semi-liquid formation and has a complex echostructure with internal reflections and unclear contours. Later, due to autolytic processes and sedimentation of a suspension from blood and pus, clear signs of liquid contents appear and a false capsule with smooth walls is formed. Infection of pseudocysts often occurs, then internal echo structures or thin delicate septa can be detected. When a cyst is detected, it is important to trace the connection of the cyst with the duct, as this is important for determining treatment tactics. When a pseudocyst is more than 10 cm in size, difficulties arise in determining its source.
| Photo. A - Large pseudocyst between the head of the pancreas and the liver after pancreatitis. B, C - Severe necrotizing pancreatitis longitudinal (B) and transverse (C) sections: extensive necrosis, melting of the surrounding fat in the tail area, accumulation of fluid around the gland. | ||