Pseudomembranous enterocolitis is a fairly common pathology encountered in the clinical practice of proctologists and gastroenterologists. It is generally accepted that this pathological condition is a consequence of taking antibacterial drugs of various groups, as well as other medications. During antibiotic therapy, favorable conditions arise for the active reproduction of opportunistic anaerobic bacteria - clostridia. Specific dysbiosis causes inflammation of the large intestine, as well as damage to the mucous membrane of varying severity.
Detailed description of the study
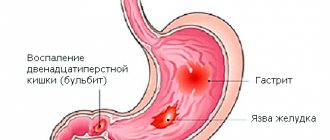
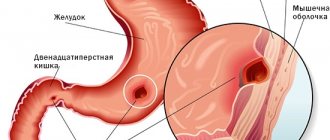
Pseudomembranous colitis is a disease of the colon caused by the bacteria Clostridium difficile. It occurs in people whose normal intestinal microflora is disrupted, usually due to recent use of antibiotics. The disease may develop against the background of long-term asymptomatic carriage of this type of clostridia or infection with this bacterium in a medical institution.
C. difficile is a spore-forming bacteria that, when overproduced, produces toxins A and B. These toxins are the main cause of the inflammatory response in the colon, which leads to increased vascular permeability and the formation of gray-yellow plaques (pseudomembranes) on the mucous membrane intestines. This condition is known as pseudomembranous colitis, or Clostridioides difficile-associated colon disease.
C. difficile is one of the most common causes of diarrhea in hospitals worldwide. This is facilitated by the transmission of infection from a sick person or carrier due to insufficient hand washing, changing bed and underwear, using shared showers and toilets, and inattention to careful personal hygiene.
The main risk factors for the development of pseudomembranous colitis are old age, severe concomitant diseases, previous use of broad-spectrum antibiotics and prolonged hospitalization. Medicines that suppress stomach acid also increase the risk of developing this disease.
Symptoms of pseudomembranous colitis include watery diarrhea, sometimes mixed with blood, abdominal pain and increased gas production. The frequency of stool depends on the severity of the disease. In addition, in severe cases, there is an increase in body temperature to 38-39 C, and there is a risk of complications in the form of pathological expansion of the intestinal area (toxic dilatation).
Laboratory diagnosis of pseudomembranous colitis is based on the detection of C. difficile toxins A and B in the stool. This is a reliable way to identify waste products of bacteria, which does not require complex manipulations to collect biomaterial, which helps to quickly prescribe optimal treatment.
Treatment of pseudomembranous colitis
The main preventive measure to prevent pseudomembranous colitis is the reasonable prescription of any antibacterial therapy. Among other things, if there is a need for treatment with antibiotics, then simultaneous use of medications that prevent the development of intestinal dysbiosis is necessary. Such drugs should be prescribed by the attending physician, taking into account all the individual characteristics of the patient’s body. Antibacterial therapy is prescribed with particular caution to those patients with a history of acute or chronic gastrointestinal diseases.
Antibiotics should also be prescribed with caution to patients over 65 years of age and to those patients taking various histamine receptor antagonists. In this category, it is necessary to evaluate the expected benefit for the patient from taking antibiotics and the potential risk of developing pseudomembranous enterocolitis.
As a rule, treatment is prescribed conservatively. First of all, antibiotics are stopped and a specific diet is prescribed. Drinking plenty of fluids is necessary to prevent the development of dehydration. For clinical forms of moderate severity and in severe patients, metronidazole or its analogs are used. In mild cases, such treatment may not be relevant.
In severe clinical cases, patients are prescribed droppers, the purpose of which is to correct the water-salt balance, protein loss, and general intoxication phenomena. In some cases, surgery may be required - resection of the affected part of the large intestine. The most dangerous condition with pseudomembranous enterocolitis is intestinal perforation, which requires emergency surgical intervention with removal of part of the intestine, washing of the abdominal cavity and its drainage.
References
- Ivashkin V.T., Yushchuk N.D., Maev I.V., Lapina T.L., Poluektova E.A., Shifrin O.S., Tertychny A.S., Trukhmanov A.S., Sheptulin A. O.A., Baranskaya E.K., Lyashenko O.S., Ivashkin K.V. Recommendations of the Russian Gastroenterological Association for the diagnosis and treatment of Clostridium difficile-associated disease. Russian Journal of Gastroenterology, Hepatology, Coloproctology. 2016
- Shelygin Yu.A. et al. Clinical guidelines for the diagnosis, treatment and prevention of Clostridium difficile-associated diarrhea. Clinical recommendations. — Moscow, 2021.
- Hassoun Ali. Clostridium difficile associated disease BMJ 2018
Introduction
Doctors quite often encounter diarrhea developing in patients during or after the use of antibiotics. In Russia, both patients and a number of doctors tend to associate its appearance with “dysbacteriosis.” Foreign experts in this case talk about “antibiotic-associated diarrhea.” Clostridium difficile is the causative agent of the most severe forms of this complication, including the development of fulminant colitis and toxic dilatation of the colon. According to Canadian researchers, the incidence rate associated with infection with this pathogen ranges from 30.8 to 40.3 cases per 100 thousand patients (in hospitals with more than 200 beds) [1]. 10% of patients receiving antibiotics develop diarrhea, but only 1% develop pseudomembranous colitis.
Pseudomembranous enterocolitis and its treatment.
But what to do if, nevertheless, the antibacterial drug caused the appearance of pseudomembranous enterocolitis?
First of all, the disease should be verified.
The second is to provide high-quality treatment.
Only severe pseudomembranous enterocolitis, in which severe diarrhea has caused severe dehydration and increased intoxication of the body, requires treatment in a hospital with the use of antibacterial drugs!? Yes Yes! All the same antibacterial drugs, but capable of destroying Clostridium difficile.
Traditionally, metronidazole (orally or intravenously) or the glycopeptide vancominin are used. The possibility of using modern macrolides or fluoroquinolones, highly effective and relatively safe antibacterial agents, for these purposes is currently being discussed. The course of treatment with antibacterial drugs usually does not exceed two weeks.
The second stage of treatment for pseudomembranous enterocolitis is the administration of ion exchange resins (cholestipol, cholestyramine) or other sorbents up to 4.0-8.0 g per day for 1-2 weeks. The main goal is to remove Clostridium difficile toxins from the digestive tract. Currently, the advisability of using these drugs is not recognized by all specialists.
The third and longest stage of treatment for pseudomembranous enterocolitis is the administration of probiotics, drugs consisting of a sufficiently large number of microorganisms that must satisfy the following requirements: remain stable and viable during storage, and when entering the digestive tract, they must survive in the intestinal environment, and while having a positive effect on the human body. Currently, not only strains of lactic acid bacteria, but also varieties of bacilli (Bacillus), bifidobacteria (Biftdobactcria), enterococci (Enterococci), and yeast fungi (Saccharomyces boulardii) claim to be probiotics. In addition to living microorganisms, their various forms are used for medicinal purposes: “dried and frozen”, simply “dried”, “dissolved in milk”, killed by heating, as well as various combinations thereof. Considering the great popularity of probiotics among the population not only as a means of treating and preventing intestinal diseases, but also in the “healing” of the body (which is actively “fueled” by advertising), we will dwell on various aspects of their use in more detail.
There are potentially three different routes for administering probiotics:
- through the rectum retrogradely into the colon,
- internally as part of dairy products (yogurt, lactic acid products),
- orally in enteric capsules containing “dried” live bacteria.
It would seem that the first route of introducing probiotics is more natural and allows the use of the full range of necessary microorganisms. But it has not received sufficient scientific justification, and is also associated with certain difficulties for both the doctor and the patient, including the risk of infection by pathogenic microorganisms, including HIV infection.
The use of dairy products can also cause a number of difficult-to-solve problems, such as their limited shelf life even when stored in a cold place (which is also not always possible), the need to take a sufficiently large volume, which can often cause nausea, heaviness in the stomach (especially relevant for elderly patients, who for the most part consume a fairly small amount of food). That is why the third, capsule route of introducing probiotics into the body is most often used.
Along with the properties already listed, probiotics must meet the following requirements:
- When used together with antibacterial agents in the treatment of intestinal infections or for the prevention of antibiotic-induced diarrhea, microorganisms must be resistant to antibiotics
- Do not transfer to pathogenic flora your natural or acquired (if ingested) resistance to antibiotics
- At the pharmacodynamic level, demonstrate mechanisms of action that could explain the effectiveness of the probiotic in vitro (in vitro) and in the body (in vivo)
- Confirm its therapeutic and preventive effectiveness in well-planned (multicenter randomized, placebo-controlled, double-blind) studies that meet the requirements of evidence-based medicine.
Unfortunately, most “candidates” for the title of probiotics do not fully satisfy such stringent requirements that modern medicine uses in assessing the effectiveness of drugs.
So lactic acid bacteria are destroyed under the influence of hydrochloric acid contained in the stomach and bile found in the intestines. This primarily concerns the bacteria Lactobacillus bulgaricus and S.thermophilus , which have weak resistance to the above-mentioned aggressive environments, while Lactobacillus acidophilus and bifidobacteria are more resistant, although there is a very big difference between their strains. The substrate with which lactic acid bacteria are introduced is very important. Lactobacillus acidophilus contained in milk has the greatest resistance to gastric hydrochloric acid, somewhat less in yogurt, and even less in buttermilk.
Despite the ability of a number of microorganisms in laboratory conditions (in vitro) to suppress the growth of gram-positive and gram-negative bacteria and destroy them through the production of hydrogen peroxide, it has not yet been possible to prove a similar effect in a living organism (in vivo). Strain LA 1 Lactobacillus acidophilus in vitro is capable of sticking to cells (adhesion), which are similar to those lining the intestinal mucosa. Thus, the probiotic prevents the attachment of pathogenic microorganisms (E. coli and Salmonella typhimurium) to the cells of the intestinal mucosa, as well as the penetration of toxic E. coli, Yersinia pseudotuberculosis and Salmonella typhimurium into them.
It should be noted that this property of strain LA 1 Lactobacillus acidophilus manifests itself only in the absence of pathogenic microbes in the intestines, i.e. The probiotic is effective only as a preventative, not a therapeutic agent. The attempt to create excessive concentrations of lactobacilli that could compete with pathogenic microorganisms has not yet been successful because very high concentrations of the microorganism are required to achieve a level of protection against the adhesion of intestinal toxic E. coli (the most common cause of the disease known as traveler's diarrhea). , tens of times higher than those traditionally used.
The theoretically substantiated ability of some lactobacilli (Lactobacillus casei GG and Lactobacillus casei rhamnosus) to exert an activating effect on the immune system, thereby preventing the development of infectious diseases, has not received serious scientific confirmation. Lactobacillus acidophilus, for example Lactobacillus acidophilus, when added to milk, does not improve the absorption of lactose (milk sugar), which is included in some “medicinal” products. Therefore, in patients with a congenital deficiency of lactase, the intestinal enzyme that metabolizes lactose, consumption of such products will not only eliminate diarrhea, but may also increase it.
In addition, lactobacilli introduced as a probiotic can cause an increase in the intraintestinal concentration of lactic acid with a decrease in pH in the intestine (metabolic D-lactic acidosis), which can negatively affect the patient’s condition. This condition is further aggravated by irrational antibiotic therapy, which stimulates the growth of gram-positive bacteria that are resistant to prescribed drugs and produce D-lactate.
Another problem that may arise during treatment with drugs containing certain strains of enterococci and bacilli is the possibility, even theoretical, of the development of septic conditions (endocarditis, meningitis, pneumonia). There are reports in which these diseases developed in weakened patients with cancer and immunodeficiency who took Enicrococcus faccium and Bacillus suhtiiis as probiotics. Among the possible causes of these situations, experts discuss the possibility of the participation of bacterial plasmids that are part of these microorganisms in the cross-transmission of resistance to various antibiotics, including vancomycin, to intraintestinal microorganisms (see above).
On the other hand, most strains of Lactobacilli, Entcrococci, Bacillus cereus and Bifidobacteria are sensitive to antibiotics such as amoxicillin (semi-synthetic penicillin), doxycycline (tetracycline), fluoroquinolones, and cephalosporins. Therefore, when they are used together, one simply cannot expect normalization of the intestinal microflora, even with parenteral (intramuscular or intravenous) administration of antimicrobial agents.
Clinical trials conducted at the end of the last century for the most part did not find significant effectiveness of drugs consisting of Lactobacilli, Entcrococci, Bacillus and Bifidobacteria in eliminating the clinical manifestations of diarrhea, including those caused by pseudomembranous enterocolitis. It should be noted that we are talking specifically about medicines, and not about the numerous dairy products containing these microorganisms, which should be considered only as food additives. According to international standards, their advertising should not use medical terminology, such as the appropriateness of use in the treatment and/or prevention of diseases or their symptoms. Thanks to this, any consumer should clearly know what is a drug and what is a food product or food additive.
Standing somewhat apart from the listed microorganisms are yeast fungi ( Saccharomyces boulardii ), which are produced in frozen-dried form. When they enter the intestines, they demonstrate not only high viability, but also therapeutic and prophylactic properties against many diseases accompanied by diarrhea, which is confirmed by well-planned and conducted scientific studies. Saccharomyces boulardii is not inactivated under the influence of hydrochloric acid of the stomach and, after ingestion, is found alive in all parts of the digestive tract. After stopping use during the first 5 days, the mushrooms disappear from the intestines. Unlike previously described microorganisms, Saccharomyces boulardii is naturally resistant to most antibiotics, while maintaining sensitivity to non-absorbable antifungal drugs (for example, nystatin). As for modern absorbable antifungal drugs (fluconalol, etc.), a dosage interval of 6 or more hours no longer affects the activity of Saccharomyces boulardii. An interesting property of Saccharomyces boulardii is an increase in their content in the intestines in response to disruption of its natural microflora as a result of taking antibiotics.
The therapeutic effect of Saccharomyces boulardii against pathogenic microflora, or more precisely Clostridium difficile, is associated with the ability to compete with clostridia for monosaccharides, a necessary component of the metabolism of this microorganism. In addition, Saccharomyces boulardii produce specific enzymes (proteases) that eliminate the pathogenic effect of Clostridium difficile toxin on intestinal cells, which is accompanied by a decrease in their permeability to clostridia metabolites and suppression of fluid secretion into the intestinal lumen, thereby reducing the severity of pseudomembranous enterocolitis and its the main symptom is diarrhea. The additional therapeutic effect of Saccharomyces boulardii is explained by their ability to improve the nutrition of the cells of the intestinal mucosa and increase their secretion of immunoglobulins (protein substances that protect the body from infection).
It is thanks to these properties that Saccharomyces boulardii demonstrates its effectiveness in the treatment of acute and exacerbations of chronic forms of pseudomembranous enterocolitis caused by irrational antibiotic therapy, as well as its prevention. In addition, Saccharomyces boulardii demonstrates high effectiveness in the treatment and prevention of infectious diarrhea of other nature (for example, “travelers' diarrhea”).
Traditionally, a drug containing Saccharomyces boulardii for the treatment and prevention of infectious diarrhea, including that caused by Clostridium difficile, is prescribed 1-2 capsules 2 times a day for 1-2 weeks. There are situations when the duration of taking the drug increases to 3-4 weeks.
CAUSES OF THE DISEASE
The main reason is the use of antibacterial drugs (penicillins, cephalosporins, ofloxacin, levofloxacins, etc.). The risk of developing membranous colitis increases:
- when taking several antibiotics at the same time;
- during intensive therapy;
- when diagnosing tumors using certain medications;
- in treatment that includes the use of drugs that reduce the activity of the immune system;
- when using additional antidiarrheal drugs, non-steroidal anti-inflammatory drugs (NSAIDs), cytostatics and neuroleptics.
In addition, provoking factors include:
- chronic intestinal diseases;
- diagnosed oncology;
- circulatory disorders in the gastrointestinal tract;
- operations on the gastrointestinal tract;
- Frequent diagnostic procedures such as colonoscopy or sigmoidoscopy.
Literature
- Alfa MJ, Dul T., Beda G. Survival of incidence of Clostridiulm difficile infection in Canadian hospitals and diagnostic approaches// J. Clin. Microbiol. 1998. V. 36. P. 2076-2080.
- Barbut F., Kajzer C., Planas N., Petit JC Comparison of three enzyme immunoassays, a cytotoxicity assay, and toxigenic culture for the diagnosis of Clostridium difficile-associated diarrhea. J Clin Microbiol 1993;31:963-7.
- Bartlett JG ANTIBIOTIC-ASSOCIATED DIARRHEA N Engl J Med, Vol. 346, No 56: 334-339.
- Dallal MR Fulminant Clostridium difficile: An Underappreciated and Increasing Cause of Death and Complications. Ann Surg. March 2002; 235(3): 363–372.
- Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. Am J Gastroenterol 1997;92:739-50.
- George RH, Symonds JM, Dimock F., et al. Identification of Clostridium difficile as a cause of pseudomembranous colitis. BMJ 1978;1:695.
- Hall IC, O'Toole E. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child 1935; 49:390-402.
- Jacobs J., Rudensky B., Dresner J., et al. Comparison of four laboratory tests for diagnosis of Clostridium difficile–associated diarrhea. Eur J Clin Microbiol Infect Dis 1996;15:561-6.
- Johnson S, Kent SA, O'Leary KJ, et al. Fatal pseudomembranous colitis associated with a variant Clostridium difficile strain not detected by toxin A immunoassay. Ann Intern Med 2001;135:434-8
- Kelly CP, Pothoulakis C, LaMont JT Clostridium difficile colitis. N Engl J Med 1994;330:257-62
- Kelly CP, LaMont JT. Clostridium difficile infection. Annu Rev/Med 1998;49:375-90.
- Kreutzer EW, Milligan FD Treatment of antibiotic-associated pseudomembranous colitis with cholestyramine resin. Johns Hopkins Med J 1978;143:67-72.
- Malnick S., Zimhony O. Treatment of Clostridium difficile–Associated Diarrhea. Ann Pharmacother 2002;36:1767-75.
- Manabe YC, Vinetz JM, Moore RD, Merz C., Carache P., Bartlett JG Clostridium difficile colitis: an efficient clinical approach to diagnosis. Ann Intern Med 1995;123:835-40.
- Mylonakis E., Ryan ET, Calderwood SB Clostridium difficile–associated diarrhea. A review. Arch Intern Med 2001;161:525-33.
- Staneck JL, Weckbach LS, Allen SD, et al. Multicenter evaluation of four methods for Clostridium difficile detection: immunoCard C. difficile, cytotoxin assay, culture, and latex agglutination. J Clin Microbiol 1996;34:2718-21.
- Teasley DG, Gerding DN, Olson MM, et al. Prospective randomized trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhea and colitis. Lancet 1983;2:1043-1046.
- Wenisch C., Parschalk B., Hasenhundl M., Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis 1996;22:813-8.
- Young G., McDonald M. Antibiotic-associated colitis: why do patients relapse? Gastroenterology 1986;90:1098-1099.
| back |