Symptoms Classification Diagnostics Treatment Information block
Chronic gastritis is an inflammation of the gastric mucosa that occurs under the influence of factors of various etiologies (bacterial, chemical, thermal and mechanical).
THE INSTITUTE OF ALLERGOLOGY AND CLINICAL IMMUNOLOGY has:
- range of services for accurate diagnosis of diseases
- special treatment programs
- consultations
- carried out by doctors with academic degrees.
When identifying concomitant diseases, consultations are held with specialists in related fields (otorhinolaryngologists, endocrinologists, neurologists, gastroenterologists, dermatovenerologists), consultations with Doctors of Medical Sciences.
Attention! The same symptoms can be signs of different diseases! Do not self-medicate, consult a gastroenterologist!
Symptoms
| With increased acidity | With low acidity |
| Heartburn Belching sour Dull aching pain in the epigastrium after eating. Feeling of heaviness, pressure in the epigastrium after eating. Constipation. Decreased appetite | Belching air Nausea, vomiting Burning pain in the epigastrium after eating Diarrhea Flatulence, rumbling in the stomach. |
Classification
Sydney classification system for chronic gastritis
| Type of gastritis | Synonyms (former classifications) |
| Non-atrophic | Superficial Chronic antral Gastritis type B Hypersecretory gastritis |
| Atrophic autoimmune | Type A gastritis Diffuse gastritis of the gastric body associated with B12-deficiency anemia and decreased secretion |
| Atrophic multifocal | Mixed gastritis type A and B |
| Chemical | Reactive gastritis type C Reactive reflux gastritis |
| Radiation | |
| Lymphocytic | Gastritis associated with celiac disease |
| Granulomatous | Isolated granulomatosis |
| Eosinophilic | Allergic |
| Other infectious | Bacteria (except Helicobacter pylori) Fungi, Parasites |
| Giant hypertrophic | Ménétrier's disease |
According to the clinical picture
| chronic gastritis type A | gastritis type B | type C |
| primary autoimmune gastritis of the fundus of the stomach (fundic) | antral gastritis of bacterial origin | reflux gastritis |
| By origin | According to the state of secretory function | According to histological picture | According to the anatomical location of the inflammation zone |
| bacterial, autoimmune, endogenous, iatrogenic, reflux gastritis | decreased increased normal | superficial, atrophic, hyperplastic | antral fundamental |
Volgograd
BY FREQUENCY:
Type - A: 5%,
type - B: 85%,
type - C: 10% of all patients with chronic gastritis
At age >50 years, 50% of people have chronic superficial gastritis type B.
2 classification systems for gastritis:
ABC classification of chronic gastritis:
It is based on etiological and histological criteria.
Type A (synonyms: body gastritis, autoimmune gastritis).
Features: descending distribution from the mucous cardia to the body of the stomach.
The essence of pathogenesis: this is an autoimmune disease with autoantibodies to parietal cells in 90%, as well as autoantibodies to intrinsic Castle factor in 70%.
As a result of the disappearance of parietal cells, achlorhydria develops (i.e., lack of HCL).
And due to a lack of intrinsic factor Castle, B12-deficiency anemia (or so-called pernicious anemia) may occur.
Patients with chronic gastritis of the gastric body may also have other autoimmune diseases, such as:
- Addison's disease
- Hashimoto's thyroiditis.
Type B (synonyms - gastritis of the antrum, HP-gastritis caused by Helicobacter pylori)
Helicobacter population density determines:
- the degree of gastritis, which depends on the infiltration of the mucous membrane by lymphocytes and plasma cells,
- and gastritis activity, which depends on the infiltration of the mucosa by neutrophilic granulocytes.
Features: type B gastritis has an ascending spread, as a result of which the antrum/corpus border moves upward, a decrease in the number of parietal cells occurs and hypochlorhydria develops, BUT!!! Against the background of hypochlorhydria, achlorhydria never occurs.
The pathogenesis is based on: infection of the gastric mucosa by Helicobacter Pylori: gram-negative bacteria with high urease activity, which creates the possibility of rapid confirmation using express methods.
Type C (synonyms - chemically induced gastritis):
It is based on gastritis caused by bile reflux, which can develop, for example, in the gastric stump after Billroth-I or Billroth-II surgery.
Rare special forms of gastritis, for example:
Crohn's gastritis: gastric manifestations of Crohn's disease: continuous infiltration of the gastric mucosa by granulocytes + confirmation of epithelial cell granuloma. Therefore, even if this pathology is suspected, it is necessary to diagnose the lower digestive tract for Crohn’s disease, and mandatory delayed FCS.
Classification of gastritis according to Sidney (1990):
It is based on etiological, histological and endoscopic criteria.
Etiology: as with ABC - classification of gastritis.
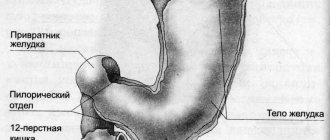
Topographically:
- gastritis of the antrum,
- body gastritis,
- pangastritis
A. Endoscopic categories of gastritis:
- Erythematous (exudative) gastritis,
- Gastritis with flat erosions,
- Gastritis with polypoid erosions,
- Atrophic gastritis (folds of the mucous membrane are smoothed or completely disappeared),
- Hemorrhagic gastritis,
- Reflux gastritis (reflux of bile into the stomach),
- Gastritis with giant folds.
B. Histological parameters of gastritis:
morphology includes 5 parameters:
I. Chronic gastritis: indicators:
- infiltration of the lamina propria by lymphocytes and plasmacytes (which are not found in the normal gastric mucosa),
- formation of lymphatic follicles
- Based on the size of lymphoplasma cell infiltration, 3 degrees of severity are distinguished.
II. The activity of the inflammatory process: correlates with the density of neutrophil granulocytes, and 3 degrees of activity are distinguished.
III. Atrophy of the body of the stomach: reduction of specific glands of the stomach, these include the main and parietal cells. Atrophic gastritis in the fundus and body of the stomach is typical for autoimmune gastritis.
IV. Intestinal or intestinal metaplasia is divided into 3 types:
Type I: complete intestinal metaplasia, transformation according to the type of small intestinal mucosa,
Type II: incomplete intestinal metaplasia with evidence of goblet cells.
Type III: incomplete metaplasia of colic or enterocolic type
V. Colonization by Helicobacter pylori (HP).
Modified Sydney CG Classification System.
| Type of gastritis | Synonyms | Etiological factors |
| Non-atrophic | Superficial, diffuse antral chronic antral, interstitial, | H. pylori type B, other factors |
| Atrophic: Autoimmune Multifocal | Type A, diffuse gastric body, associated with pernicious anemia | Autoimmune H. pylori, nutritional characteristics, environmental factors |
| Special forms: Chemical Radiation Lymphocytic Non-infectious granulomatous Eosinophilic Other infectious | Reactive reflux gastritis, type C Varilomorphic, associated with celiac disease Isolated granulomatosis Food allergies, other allergens | Chemical irritants, bile, NSAIDs Radiation injuries Idiopathic, immune mechanisms, gluten, H. pylori Crohn's disease, sarcoidosis, Wegener's granulomatosis, foreign bodies, idiopathic Allergic Bacteria (except H. pylori), viruses, fungi, parasites |
LABORATORY INDICATORS:
The level of serum gastrin in gastritis of the gastric body is high or very high, depending on the decrease in HCL production.
With antrum gastritis (the site of gastrin secretion), the gastrin level, on the contrary, is relatively low.
COMPLICATIONS:
- Recurrent peptic ulcer based on Helicobacter gastritis.
- Pernicious anemia - otherwise B12-deficiency anemia in type A gastritis.
- Increased incidence of gastric carcinoma in atrophic gastritis type. And also with HP gastritis with intestinal metaplasia.
- Increased risk of developing primary gastric lymphoma of the MALT type in Helicobacter gastritis.
MALT - Mucosa associated lymphoid tissue - mucosa-associated lymphoid tissue
DIAGNOSIS:
- Gastroscopy with biopsy from the antrum and corpus of the stomach + examination for Helicobacter.
- Diagnosis of Helicobacter pyiori(HP): Endoscopic biopsy: rapid test for urease, histology, cytology, culture seeding,
- 13C - or 14C - breath test: after the administration of radioactively labeled urea, which forms its own urease under the influence of Helicobacter, labeled CO2 is measured in the exhaled air.
- Serological (confirmation of antibodies). After successful treatment, the antibody titer decreases by approximately 50% from the initial level within six months.
- Delineating diagnostics for type A gastritis: Autoantibodies against parietal cells, Autoantibodies against intrinsic Castle factor,
- Serum vitamin B12 levels.
ATTENTION!!! The diagnosis of chronic gastritis can be made and diagnosed only endoscopically with histological analysis.
The same applies to HP diagnostics.
Causes
| Type of gastritis | Etiological factors |
| Non-atrophic | Helicobacter pylori Other factors |
| Atrophic autoimmune | Immune mechanisms |
| Atrophic multifocal | Helicobacter pylori Nutritional disorders Environmental factors |
| Special forms | |
| Chemical | Chemical irritants: bile (DGR), NSAIDs |
| Radiation | Radiation damage |
| Lymphocytic | Idiopathic Immune mechanisms Gluten |
| Granulomatous | Crohn's disease Sarcoidosis Granulomatosis Wegener's Foreign bodies Idiopathic |
| Eosinophilic | Food allergies Other allergens |
| Other infectious diseases | Bacteria (except Helicobacter pylori) Fungi, Parasites |
| Giant hypertrophic | Ménétrier's disease |
| Exogenous (external) | Endogenous (internal) |
| 1. Diet disorder: large gaps between meals, late and night dinners, fast food, swallowing poorly chewed food. 2. Intake of foods that irritate the gastric mucosa: spicy, smoked, sour, salty, rough food, strong coffee, alcohol, scalding hot or cold food, consumption of poor quality products. 3. Chemicals and drugs. 4. Smoking. 5. Psychologically severe, stressful conditions. 6. Harmful production (high smoke or dusty atmosphere in the workplace). | 1.Helicobacter pylori (HP) 2.Parasitic infections 3. Severe infectious diseases (tuberculosis, etc.). 4.Endocrine pathologies (diabetes mellitus, thyroid pathologies). 5.Immunity disorders. 6. Impaired breathing or pathologies of the circulatory system. 7. Duodenogastric reflux. |
University
→ Home → University → University in the media → Variants of the course of chronic gastritis
The photo is for illustrative purposes only. From the MV archive
Ivan Bronovets, Professor of the Department of Cardiology and Internal Medicine of BSMU, Doctor of Medicine. sciences
Chronic gastritis (CG) is a recurrent focal or diffuse inflammation of the mucous and submucosal membrane of the stomach, accompanied by impaired regeneration, with a tendency to progression, the development of atrophy and secretory failure. The main changes occur in the mucous membrane, so the functional morphology of the mucosa, and not the entire stomach, is studied.
Chronic gastritis occupies a leading place among diseases of the digestive system. Various variants of the pathology are diagnosed in 50–75% of patients over 45 years of age.
Classification
The Sydney classification of gastritis (1990), modified in Houston (1994), has received general recognition. It combines etiological, topographical and morphological principles for assessing the disease. According to the etiology, CG is divided into Helicobacter pylori (type B), autoimmune (type A), chemical or reflux gastritis (type C) and special forms (lymphocytic, radiocytic, granulomatous, eosinophilic gastritis, etc.).
Topographically, gastritis is distinguished between the body of the stomach, antrum, fundus and/or all parts of the stomach (pangastritis). Based on morphological characteristics, the presence and degree of inflammation, atrophy, metaplasia, and contamination with Helicobacter pylori (HP) bacteria are assessed. Gastritis is divided into erosive and non-erosive. CG can be superficial and deep (transmural).
In addition to the main etiological factors in the development and progression of chronic hepatitis, poor diet, rough food, abuse of spices, alcohol, smoking, systematic trauma to the gastric mucosa with poorly chopped food, hasty eating, etc. play a role.
Helicobacter pylori gas mainly affects the antrum of the stomach. HP is most often detected on the surface epithelium under a layer of mucus (superficial gastritis) and much less frequently in atrophic gastritis.
The main morphological feature of this type of gastritis is the presence of HP in the bicillary form and in the form of cocci on the mucous membrane. The surface epithelium is affected, and leukocyte infiltration appears. The determination of HP and the degree of contamination of the antrum are important in diagnosis. The gold standard for diagnosis is the examination of biopsy specimens not only from the antrum, but also from the area of the body of the stomach.
Autoimmune CG is much less common than HP-associated. Diagnosed in 16% of patients with macrocytic hyperchromic (pernicious) anemia. A characteristic feature is the localization of changes in the fundus of the stomach, while the antrum remains unaffected.
This leads to a sharp decrease in acidity and internal factor. Intrinsic factor deficiency causes malabsorption of vitamin B12 and the development of pernicious anemia. Severe atrophic gastritis develops with progressive loss of parietal cell mass.
It proceeds unsystematically until one of the complications develops (vitamin B12 deficiency or malignant lesions of the stomach); metaplasia appears. At the final stage, complete metaplastic replacement of the mucous membrane is noted. Antibody titers to parietal cells and intrinsic factor and achlorhydria are detected.
Reflux gastritis is associated with the reflux of duodenal contents into the stomach, damaging its mucous membrane. True reflux gastritis develops in patients who have undergone gastrectomy.
A synonym for reflux gastritis is alkaline gastritis. It is not entirely accurate, since the main role in damage to the gastric mucosa is played not by the alkaline properties of the intestinal contents, but by the characteristics of bile acids. In addition, a necessary condition for the development of gastritis is the presence of hydrochloric acid.
The main component of duodenal contents is bile acids, their salts, pancreatic enzymes and lysolecithin. Bile acids cause damage to the protective gastric mucus barrier by increasing the back diffusion of hydrogen ions, and when gastric secretion is low, pancreatic enzymes can also damage the gastric mucosa.
In case of chronic hepatitis, especially progressive one, metaplasia of the gastric mucosa of the intestinal type often occurs. We are talking about replacing the gastric epithelium with intestinal epithelium. With atrophic gastritis and gastric cancer, metaplasia is observed in almost 100% of cases. Currently, it is customary to distinguish two types of metaplasia: complete, resembling the small intestine, and incomplete (large). The presence of Paneth cells is the most important sign of complete intestinal metaplasia.
Diagnostics
To make a clinical diagnosis, it is necessary to correctly diagnose the type of hCG, assess the degree and prevalence of atrophy and other morphological signs of the disease. Dysregeneration determines the morphogenesis and clinical and morphological picture of chronic hepatitis.
The presence of chronic inflammation can only be discussed when it is detected during a morphological study. Before the biopsy, the clinician has the right to diagnose CG and can only use the syndromological designation “non-ulcer dyspepsia”.
The endoscopic research method makes it possible to determine the diagnosis with great accuracy (or significantly reduce the number of proposed diagnoses), examine all parts of the stomach, and take biopsies for histological examination.
The possibilities of endoscopic examination are not limited only to visual examination of the gastric cavity and taking biopsies; there are a number of additional techniques used in conjunction with endoscopy (chromogastroscopy, transendoscopic pH-metry). Gastroscopy with a complete clinical and morphological examination of biopsy samples of the gastric mucosa (with determination of Hp) is considered the correct method for diagnosing various types of gastric lesions.
The most common cause (70–80%) of chronic gastritis is the bacterium Helicobacter pylori. Autoimmune gastritis is diagnosed in 15–18% of cases, about 10% are due to NSAIDs, less than 5% are reflux gastritis, 1% are rare forms of hCG.
Photo: Evgeniy Krech Medical Bulletin , October 5, 2017
Share
Diagnostics
Urease breath test is the most innovative and accurate test for Helicobacter pylori! The best method for quality control of treatment!
THE INSTITUTE OF ALLERGOLOGY AND CLINICAL IMMUNOLOGY has a fundamental base that allows it to carry out a full range of diagnostic and therapeutic measures that meet the level of international standards. Many of the diagnostic and treatment methods were developed by the staff of our Institute.
The diagnosis is established on the basis of the patient’s clinical picture and history, physical examination, laboratory diagnostics, endoscopy (gastroscopy), biopsy and other studies.
If necessary, you can be advised by related specialists and experienced doctors who are developing new diagnostic and treatment methods in these areas of medicine.
Every patient with chronic gastritis must remember that gastritis is the cause of the development of other pathologies of the gastrointestinal tract!
Laboratory diagnostics are aimed at identifying the cause of chronic gastritis, determining the level of gastrin, pepsinogen (the ratio of pepsinogen I and pepsinogen II) in the blood, and the content of vitamin B12 in the serum. Bacteriological examination, biochemical examination and other studies may be carried out.
Chronic gastritis: modern concepts, principles of diagnosis and treatment
Ivashkin V.T., Lapina T.L.
“Chronic gastritis” or “ chronic gastroduodenitis ” is a diagnosis that is extremely often implemented in medical practice, and its formulation does not always reflect the essential aspect of the disease in a particular patient. We can say that this is an “on-duty” diagnosis for a patient who consulted a doctor about dyspeptic complaints and who did not have an ulcer during endoscopy. In recent years, medical science has made a significant step forward, allowing us to reconsider our ideas about gastritis at a qualitatively new level of knowledge.
The discovery of a new type of microorganism - the bacterium Helicobacter pylori (H. pylori) - and the study of its role in human pathology was of decisive importance. It has become obvious that this microbe, which colonizes the gastric mucosa, is the causative agent of the most common variant of gastritis. Indeed, it was the establishment of the etiological significance of H. pylori that made chronic gastritis a clearly defined and clinically significant nosological entity, transformed it from an amorphous concept into a disease with a known cause, traceable stages of pathogenetic development, a definite prognosis, and, finally, created the possibility of etiotropic treatment. Thanks to H. pylori, classical ideas about gastritis have acquired greater stability, since this piece of knowledge made it possible to balance the available information about morphological and functional changes (acid production, mucus formation, etc.) and the connection of gastritis with other diseases, primarily peptic ulcer and stomach cancer [2,3,4].
The second significant step was the development of a modern classification of gastritis. The so-called Sydney system was proposed in 1990 and expanded in 1994. Since then, although not without its shortcomings (like any classification), the Sydney system has been recognized almost throughout the world. It turned out to be convenient in everyday diagnostic practice and provided a unified conceptual approach and language in describing changes in the stomach. An unconditional positive quality of the classification should be considered the proposed visual analog scale for semi-quantitative determination of the activity and severity of gastritis, atrophy and intestinal metaplasia, the degree of H. pylori colonization, which significantly reduced the subjectivity of assessments when characterizing the histological picture.
Chronic gastritis caused by H. pylori infection
- Frequency
H. pylori infection is of global significance and is widespread, including in our country, where, according to epidemiological data, more than 80% of the adult population are infected. H. pylori causes inflammatory changes in the gastroduodenal mucosa in almost all infected individuals, which are the actual substrate of gastritis. Helicobacter gastritis is the most common type of gastritis. Thus, about 5% of all patients with gastritis suffer from autoimmune gastritis, another 5% - from other special forms of gastritis, which will be discussed below. Thus, inflammation of the gastric mucosa caused by H. pylori accounts for 90% of all forms of gastritis.
- H.pylori is an etiological factor of chronic gastritis
After taking the culture of H. pylori per os, it was possible to reproduce the occurrence of morphological changes typical of gastritis in the previously normal mucous membrane in self-infection experiments, one of which was carried out by the discoverer of the bacterium, B. Marshall. Studies are being conducted on laboratory animals in which the formation of gastritis under the influence of the causative bacterium is reproduced.
Infection with H. pylori leads to the appearance of an inflammatory infiltrate in the gastric mucosa, and the presence of H. pylori is always accompanied by morphological signs of gastritis. Infiltration by polymorphonuclear leukocytes (neutrophils) is directly caused by a bacterium that secretes a special protein that activates neutrophils. The gene encoding its synthesis (napA) has been identified in all strains of H. pylori. Thus, this microorganism has the specific ability to cause neutrophilic infiltration of the gastric mucosa, which characterizes the activity of gastritis. Adhesion of H. pylori to gastric epithelial cells, as well as adhesion in the case of any other bacterial infection of the mucous membrane, causes reorganization of the cytoskeleton of epithelial cells and a number of other changes; epithelial cells respond to this by producing cytokines—interleukin-8 (IL-8) and some other chemokines. These cytokines also lead to the migration of leukocytes from the blood vessels, and the active stage of inflammation develops. Activated macrophages secrete interferon-g and tumor necrosis factor. These factors sensitize the receptors of lymphoid, epithelial and endothelial cells, which in turn attracts a new wave of cells involved in immune and inflammatory reactions to the mucous membrane. Catalase and superoxide dismutase produced by H. pylori neutralize phagocytes, allow the microbe to avoid phagocytosis and promote the death of polymorphonuclear leukocytes. Active oxygen metabolites of neutrophils have a damaging effect on gastric epithelial cells. The chronic phase of infection is characterized by significant lymphocytic infiltration and loss of epithelial integrity.
According to modern concepts, H. pylori causes a change in the normal processes of regeneration of the gastric epithelium: the microorganism causes (directly or indirectly) disregenerative processes, which serve as an important component in the pathogenesis of gastritis; H. pylori affects both the proliferation and apoptosis of epithelial cells of the gastric mucosa. It is clear that disruption of cellular renewal processes in the gastric mucosa underlies the morphogenesis of atrophy in gastritis.
Important evidence of the etiological role of H. pylori is very easy to obtain in everyday practice, since after successful treatment of the infection, regression of individual components of the morphological picture of gastritis occurs.
- Outcomes of H. pylori infection
Infection with H. pylori occurs mainly in childhood and without treatment, the persistence of the microorganism becomes lifelong. Depending on the virulent properties of H. pylori and the genetic characteristics of the macroorganism, the outcomes of H. pylori infection are different: in the majority of infected people, chronic gastritis is asymptomatic, in some patients (according to some authors, in 6% of infected people) a peptic ulcer develops, in some patients the progression of gastric changes leads to the development of stomach cancer. There is no doubt that H. pylori is associated with a rare form of gastric malignancy - lymphoma arising from mucous membrane-associated lymphoid tissue (MALT lymphoma).
The outcome of H. pylori infection depends on the type of chronic gastritis, which, in turn, is largely determined by the topography of H. pylori colonization of the gastroduodenal mucosa. Duodenal ulcer occurs against the background of gastroduodenitis, i.e. predominantly antral gastritis, and colonization of the duodenal mucosa by H. pylori is possible after the formation of foci of gastric metaplasia in it in response to acid aggression. It has been suggested that the initial centers of ulceration in duodenal ulcers are foci of metaplasia with adherent H. pylori and inflammatory changes. Stomach ulcers are formed against the background of diffuse (pan-) gastritis or gastritis predominantly of the body of the stomach, in which the mucous membrane, “weakened” by inflammation, is exposed to aggressive factors even with normal levels of acid secretion.
H. pylori infection is recognized by the WHO International Agency for Research on Cancer as carcinogenic to humans. The connection between H. pylori and gastric cancer is currently beyond doubt, and chronic gastritis is considered a link. Long-term persistence of H. pylori accompanies the progression of gastric changes: over the years, gastritis becomes atrophic. Even before the discovery of H. pylori, it was known that atrophic gastritis can be considered a precancerous disease. The significance of atrophic gastritis in carcinogenesis is determined by its frequency - it accounts for 3/4 of the structure of precancerous diseases of the stomach. It is known that the risk of developing gastric carcinoma with atrophic gastritis is 10 times higher than in the population. Further deepening of the gastric process is associated with the formation of intestinal metaplasia: the appearance of incomplete (or colonic) metaplasia is especially dangerous. It is this epithelium that, under certain conditions, can become the basis of dysplasia and malignancy. Thus, the pathogenetic cascade triggered by H. pylori and through the stages of atrophy, metaplasia and dysplasia leading to gastric cancer has been well studied and has come closer to completion thanks to the establishment of the etiological role of the bacterium for chronic gastritis.
Treatment of chronic Helicobacter gastritis
Modern therapy for chronic gastritis is etiological therapy aimed at eradicating H. pylori infection. Standards (protocols) for the diagnosis and treatment of patients with diseases of the digestive system, approved by the Ministry of Health of the Russian Federation, classify anti-Helicobacter regimens as necessary therapeutic measures for gastritis with the detection of H. pylori. However, we know that chronic gastritis associated with H. pylori is an extremely common disease. Having a basis for anti-helicobacter therapy for gastritis, it is necessary to understand which specific patients this therapy is indicated for. Recommendations for the diagnosis and treatment of H. pylori infection, adopted for the countries of the European Union in 1997 (Maastricht Consensus - I), indicated variants of gastritis with severe structural changes - with intestinal metaplasia, atrophy, as well as gastritis with erosions - as indications for eradication therapy. In the final document of the Second Consensus Conference on the diagnosis and treatment of H. pylori infection (Maastricht Consensus - II, 2000), only one form of gastritis was established as an unconditional indication for eradication therapy - atrophic gastritis.
Analysis of a large number of clinical trials allows us to identify the best regimens for the treatment of H. pylori infection that meet the requirements of evidence-based medicine. These regimens were reflected in the final document of the Second Consensus Conference on the Diagnosis and Treatment of H. pylori Infection (2000). The following three-drug regimens for at least 7 days are suggested as the initial course of treatment (first-line therapy): proton pump inhibitor (or ranitidine bismuth citrate) in a standard dose 2 times a day + clarithromycin 500 mg 2 times a day + amoxicillin 1000 mg 2 times a day or metronidazole 500 mg 2 times a day. The combination of clarithromycin with amoxicillin is preferable to clarithromycin with metronidzole, as it can help achieve a better result when prescribing second-line treatment - quadruple therapy.
If treatment is unsuccessful, the second stage begins. The patient is offered a backup regimen (second-line therapy - quadruple therapy) for at least 7 days: proton pump inhibitor at a standard dose 2 times a day + bismuth subsalicylate/subcitrate 120 mg 4 times a day + metronidazole 500 mg 3 times a day + tetracycline 500 mg 4 times a day.
In our country, triple regimens based on bismuth are widely used as first-line therapy (the best known is colloidal bismuth subcitrate). It should be noted that it is for the treatment of chronic gastritis that such regimens may be the most appropriate, since when prescribing H. pylori eradication in the event of an exacerbation of peptic ulcer disease, the need for rapid relief of pain and dyspeptic syndrome forces preference for regimens based on proton pump inhibitors. For chronic gastritis, which has no symptoms, triple regimens based on bismuth salt may be optimal in terms of cost-effectiveness ratio.
Reversibility of changes inherent in chronic gastritis under the influence of H. pylori eradication
After eradication of the infection, within a month the neutrophilic infiltration of the epithelium and the lamina propria of the mucous membrane (reflecting the activity of gastritis) completely disappears, and at a later date the mononuclear infiltration disappears. Thus, chronic non-atrophic gastritis is completely cured after the destruction of H. pylori. There are conflicting opinions in the literature regarding the possibility of reverse development of atrophy and intestinal metaplasia after eradication of infection. It is possible that the follow-up period is too limited to evaluate such changes, but there have been studies with relatively long follow-up that also showed the opposite results. Nevertheless, it seems quite reasonable that successful eradication of H. pylori, even at the stage of atrophy, leads to the interruption of the pathological cascade in the gastric mucosa and can be considered as a prevention of the development of gastric cancer. The eradication of H. pylori in patients with atrophic gastritis is supported by a number of studies (including those carried out in our clinic), which prove that the destruction of the infection normalizes the processes of cellular renewal in the gastric mucosa.
Chronic Helicobacter gastritis and functional dyspepsia
The relationship between chronic Helicobacter gastritis and functional dyspepsia
The diagnosis of gastritis is a morphological diagnosis, that is, it can be considered valid after evaluating gastrobiopsy samples by a specialist pathologist. In accordance with the requirements of the Sydney system, in order to correctly interpret the condition of the gastric mucosa, a minimum of five biopsies are required: 2 - from the antrum at a distance of 2-3 cm from the pylorus along the greater and lesser curvature, 2 - from the body of the stomach at a distance of 8 cm from the cardia along the greater and lesser curvature, 1 - from the angle of the stomach.
“Clinical” diagnosis of gastritis, i.e. diagnosis without morphological examination of gastrobiopsy samples is practically meaningless. Complaints, and these are, as a rule, symptoms of dyspepsia that force the patient to see a doctor, are functional in nature and are not caused by those morphological changes that constitute the essence of gastritis. Changes detected during esophagogastroduodenoscopy (for example, hyperemia of the mucous membrane) are subjective and can only indirectly indicate the presence of gastritis, especially the degree of its progression.
In case of complaints of pain and discomfort in the epigastric region and in the absence of ulceration during endoscopy, a syndromic diagnosis of functional dyspepsia is convenient for both the doctor and the patient. This approach allows the doctor to use the entire possible arsenal of medicines and spa factors and establish a trusting relationship with the patient, explaining the nature of his illness in functional terms.
Criteria for the diagnosis of functional dyspepsia have been established (Rome II criteria). A doctor has the right to make this diagnosis if:
- the presence of persistent or recurring dyspepsia (pain and discomfort in the epigastric region) for at least 12 weeks (not necessarily consecutive) within the last 12 months;
- the absence of an organic disease (including according to the results of esophagogastroduodenoscopy) that would explain the presence of the listed symptoms;
- the absence of relief of symptoms after defecation and the absence of their connection with changes in the frequency and nature of stool (that is, irritable bowel syndrome is excluded).
Depending on the predominant symptom, variants of functional dyspepsia are distinguished: if the leading symptom is pain in the epigastric region, they speak of an ulcer-like variant; if the dominant symptom is discomfort - a feeling of fullness in the epigastrium, early satiety, bloating, nausea - talk about the dyskinetic variant; the intermediate state was called a nonspecific variant.
Ideas about the pathogenesis of functional dyspepsia are still evolving. When establishing this diagnosis, organic diseases must be excluded - peptic ulcer, gastroesophageal reflux disease, tumors, diseases of the pancreato-biliary zone and taking medications, such as NSAIDs. However, the diagnosis of functional dyspepsia is not contradicted by the presence of chronic gastritis associated with H. pylori infection. The significance of the morphological changes inherent in gastritis for functional dyspepsia is practically unknown. According to European authors (for populations with a low prevalence of H. pylori), H. pylori infection is found in 30-70% of cases of functional dyspepsia. A meta-analysis by D. Armstrong (1996) demonstrated that H. pylori is more common in patients with dyspepsia than the average population.
Treatment of functional dyspepsia
The lack of a complete understanding of the pathogenesis of functional dyspepsia and the characteristics of patients (including psychological) make the treatment of this syndrome a difficult task.
Antacid drugs (Almagel) are used in everyday practice as first-line therapy for functional dyspepsia. Antacids are well known to patients and doctors, the safety of their use is known, so this group of drugs is always used for functional dyspepsia. Many patients start taking antacids even before consulting a doctor and will return to it in the future, including thanks to over-the-counter supplies.
Histamine H2 receptor blockers are a group of drugs that are most often used in the treatment of functional dyspepsia. The results of clinical studies are conflicting. Some authors believe that the positive results from histamine H2 receptor antagonists in a number of clinical trials were obtained due to the group of patients with gastroesophageal reflux disease who were involved in these trials due to imperfect patient selection criteria. However, the meta-analysis by G. Dopilla et al. (1989) showed that when using histamine H2 receptor blockers, the therapeutic effect was 20% better compared to placebo. There is an opinion that for functional dyspepsia a high dose of histamine H2 receptor antagonists may be effective, but serious clinical work is needed to confirm this opinion.
Proton pump inhibitors have practically not been used for functional dyspepsia. A recently completed serious clinical study with high statistical significance demonstrated the good effect of omeprazole in functional dyspepsia, and the best result was obtained in the group of patients with an ulcer-like variant of the syndrome compared to dyskinetic. In the OCAY study, omeprazole monotherapy was as effective in relieving symptoms of dyspepsia as eradication therapy for H. pylori infection.
Conflicting results have been obtained during eradication therapy for H. pylori infection in functional dyspepsia: some studies have shown an improvement in clinical symptoms after eradication of H. pylori, some studies have not found a significant positive effect after eradication. A study by J. Gillvary et al. (1997) stands out from a number of such works, since the classic triple therapy with colloidal bismuth subcitrate was used as an eradication scheme. The Maastricht Consensus II put an end to this debate and named functional dyspepsia as an indication for eradication therapy based on the following provisions: eradication of H. pylori is an acceptable choice in the treatment of functional dyspepsia; In some patients, eradication of H. pylori leads to long-term improvement in well-being.
Thus, etiotropic treatment for chronic Helicobacter gastritis does not help all patients with functional dyspepsia, which indicates the lack of identity of these diseases and the heterogeneity of patients with functional dyspepsia. Probably, inflammatory changes in the gastric mucosa are not the main cause of dyspeptic symptoms.
Non-Helicobacter gastritis
- Autoimmune gastritis
After widespread Helicobacter gastritis, the greatest clinical significance is autoimmune gastritis associated with pernicious anemia, which in the Sydney system is placed under the heading of atrophic gastritis. With this disease, in 90% of cases, antibodies to parietal cells and to H+,K+-ATPase are detected, in 60% of cases - antibodies to Castle factor, in 50% of cases - antithyroid antibodies. Severe atrophy of the fundic glands is clinically manifested by achylia, with response hypergastrinemia and G-cell hyperplasia characteristic. The risk of stomach cancer due to autoimmune gastritis is 3-10 times higher than in the general population.
- Special forms of gastritis
In the Sydney system, special forms of gastritis include chemical, radiation and infectious gastritis, which we group according to etiology, and eosinophilic, lymphocytic and granulomatous gastritis, which we group according to their morphological features and diagnosis.
These are quite rare diseases, inferior in their clinical significance to chronic Helicobacter gastritis, which, thanks to the study of H. pylori, we can now successfully recognize and treat.
References 1. Aruin L.I., Kapuller L.L., Isakov V.A. Morphological diagnosis of diseases of the stomach and intestines. Moscow, Triad-X, 1998 2. Ivashkin V.T., Uspensky V.M. Some functional and histochemical changes in the duodenum during peptic ulcer disease (according to telemetry and duodenobiopsy). // Soviet medicine, 1970, No. 3, pp. 10-14. 3. Ivashkin V.T., Dorofeev G.I. Violations of the resistance of the mucous membrane of the stomach and duodenum in chronic gastritis and peptic ulcer. // Soviet medicine, 1983, No. 2, pp. 10-15. 4. Ivashkin V.T. Eradication of Helicobacter pylori infection and remission of peptic ulcer disease: are these conditions unambiguous? // Russian Journal of Gastroenterology, Hepatology and Coloproctology, 1999, T.8, No. 3, pp. 71-73.